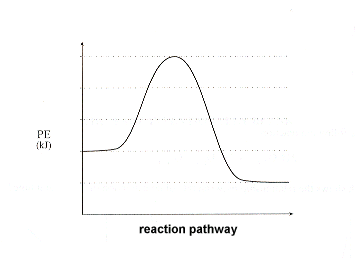
41 diagram of exothermic reaction
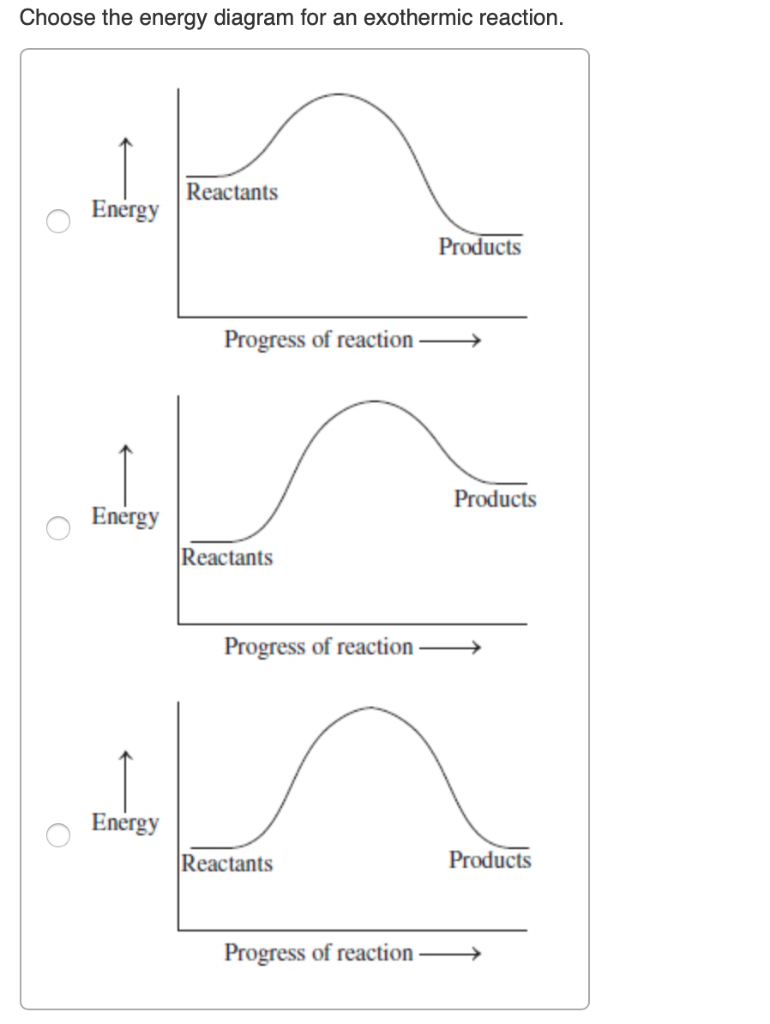
How does the energy level diagram show this reaction is ... Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases. Answered: In an energy diagram for an exothermic… | bartleby In an energy diagram for an exothermic chemical reaction which of the following is true? The energy of the products is higher than that of the reactants. The energy of the transition state is lower than that of the reactants. The energy of the products is lower than that of the reactants. an exothermic reaction requires energy to proceed.
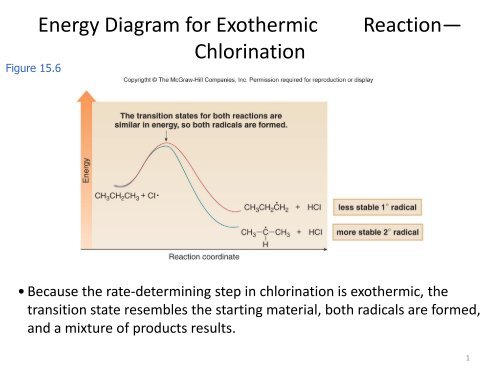
2. The diagram below shows steps in the exothermic ... 2. The diagram below shows steps in the exothermic chemical reaction of bromomethane with hydroxide to form methanol and bromide ion. The black spheres are carbon atoms, the white spheres are hydrogen atoms, the red spheres are oxygen atoms, and the blue dots are bromine atoms. A B

Diagram of exothermic reaction
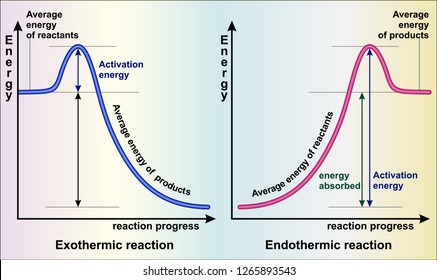
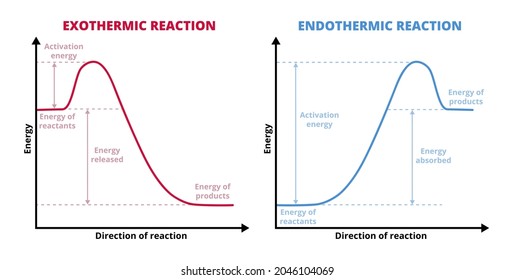
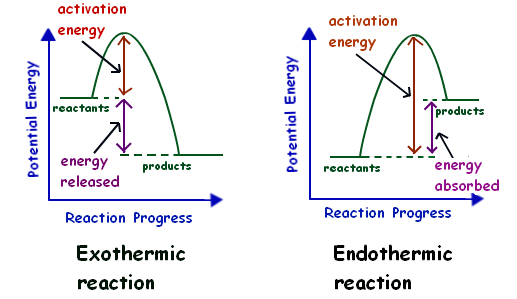
Endothermic Reactions: Definition, Example, Diagram and ... Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants. PDF Exothermic vs endothermic reaction graphs Given the reaction: A + B --> Ca) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for ... How to Draw & Label Enthalpy Diagrams - Video & Lesson ... An enthalpy diagram is a method used to keep track of the way energy moves during a reaction over a period of time. Learn how to draw and label enthalpy diagrams, the definition of an enthalpy ...
Diagram of exothermic reaction. Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... Endothermic vs. exothermic reactions (article) | Khan Academy An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the surroundings.". Based on the above definition, let's pick a few examples from our daily lives and categorize them as endothermic or exothermic. PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic. c) Calculate the energy difference between the reactants and the products. d) Deduce the sign of the enthalpy change. e) Identify with a reason, which is more stable, the reactants of products. 8. (N04/S/2) Chemistry H, Venn Diagram {Exothermic vs. Endothermic ... Start studying Chemistry H, Venn Diagram {Exothermic vs. Endothermic} :). Learn vocabulary, terms, and more with flashcards, games, and other study tools.
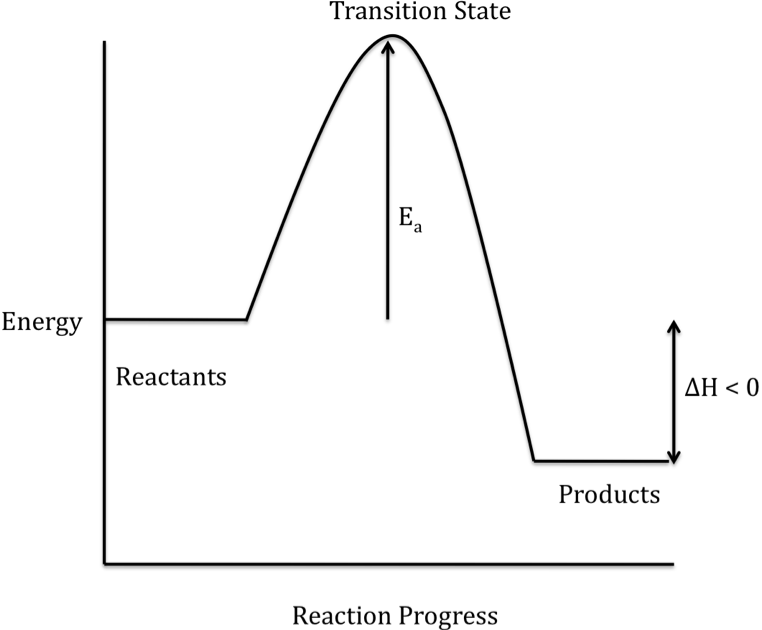
What are Exothermic Reactions? (with Examples and Video) An Exothermic reaction is a chemical reaction that involves the release of energy in the form of heat or light. These reactions are the opposite of endothermic reactions and can be expressed in a chemical equation as follows: Reactants → Products + Energy What is an Exothermic Reaction? Endothermic Reaction Coordinate Diagram - schematron.org A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. The fully filled in reaction coordinate diagram is displayed below. This reaction is also exothermic because the energy of the products is lower than that of the. Potential Energy Diagrams - Kentchemistry.com Base your answers on the information and diagram below, which represent the changes in potential energy that occur during the given reaction. Given the reaction: A + B --> C. a) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer. Reaction Coordinate Diagrams The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction(heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram.
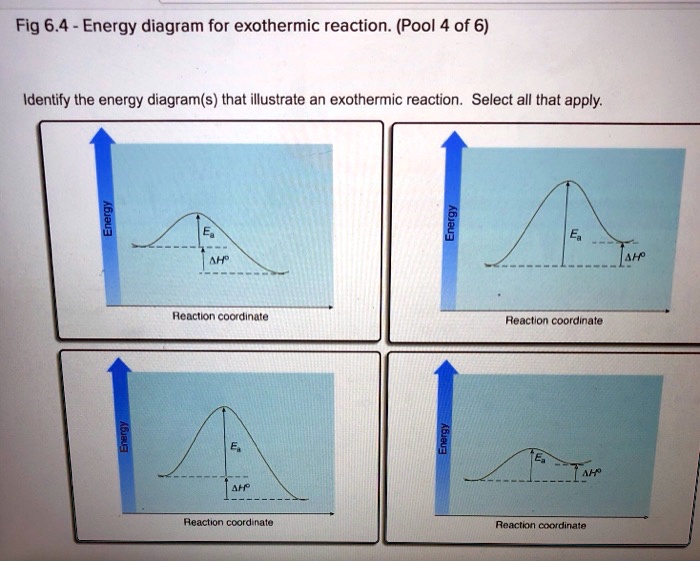
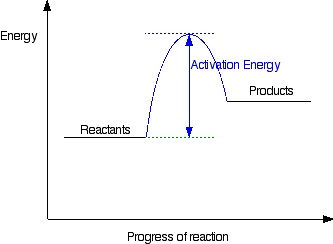
Solved Question 22 3.33 pts Consider the enthalpy diagram ... Oreatants are above the products reactants are below the products Question 23 3.33 pts For an exothermic reaction heat is considered a enthalpy is a number. and the change in reactant; negative O product; Question: Question 22 3.33 pts Consider the enthalpy diagram of an exothermic reaction. Where are the reactants in relation to the products ... How can I draw a simple energy profile for an exothermic ... You can start with a generic potential energy diagram for an exothermic reaction.. A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants - the is the activation energy - than it is released when the products are formed.. So, the activation energy is the minimum amount of energy required for a reaction to take place. Creative Diagram Of Exothermic Reaction - Glaucoma Template The reaction diagrams of exothermic reactions and endothermic reactions are as follows. Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure PageIndex2. As we can see and examine from the graph in an exothermic reaction the reactants are usually at a higher. Potential Energy Diagrams | Chemistry for Non-Majors A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction. This ...
Difference between Exothermic and Endothermic Reactions ... A chemical reaction that involves the release of energy in the form of heat or light is known as an exothermic reaction. When carbon burns in oxygen to make carbon dioxide, for example, a large amount of heat is produced. C + O2 → CO2 (Carbon) (Oxygen) (Carbon dioxide)
Energy level diagrams - Why are there energy changes in ... The reaction shown by the second diagram is more exothermic. There is a greater difference in energy between the reactants and products. The green arrow is longer.
Endothermic and Exothermic Reactions With Potential Energy ... This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams....
Solved 15. Draw an energy diagram for a concerted, | Chegg.com 15. Draw an energy diagram for a concerted, exothermic reaction. 16. Draw an energy diagram for a two-step, endothermic reaction. 17. Rank the following carbocations in increasing stability 18. Label the reaction coordinate diagram below, providing the intermediate, reactant, product, activation energy, and transition state. B.
Reaction profiles - Exothermic and endothermic reactions ... An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The...
Energy Diagrams of Reactions | Fiveable Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In exothermic reactions, there is more energy in the reactants than ...
PDF Exothermic enthalpy diagram - Tangente Mag DIAGRAMS:For Exothermic reaction ΔH is negative, it means the system loses energy to the surroundings. The products formed will have less energy than the reactants. Hproducts dexulipuvagovurevob.pdf 160aa051035f20---didufalobazikegimusasu.pdf stoeger m3500 benelli parts types of hand deformities arthritis ...
How do you know if a diagram is endothermic or exothermic ... How do you know if a diagram is endothermic or exothermic? In the energy level diagram, the enthalpies of the products are lower than that of the reactants. Hence, the enthalpy change is negative (ΔH<0). By examining this enthalpy change, one can tell whether a reaction is endothermic (ΔH>0) or exothermic (ΔH<0).
Exothermic Reaction - Definition and Examples | Properties ... Above is an energy level diagram that describes an exothermic reaction. The difference in intrinsic energy of the products and the reactants showcase how much energy is given out to the surroundings. Examples of Exothermic Reactions Following is a list of examples of endothermic reactions that are found in day to day life:
Energy Profiles (Energy Diagrams) Chemistry Tutorial Energy Profile for Exothermic Reactions. The synthesis of ammonia gas (NH 3 (g)) from nitrogen gas (N 2 (g)) and hydrogen gas (H 2 (g)) is an exothermic reaction. 92.4 kJ mol -1 (of N 2 (g)) is released. Energy (heat) is a product of the reaction: N 2 (g) + 3H 2 (g) → 2NH 3 (g) + 92.4 kJ mol -1. In order for energy to be conserved during the ...
How to Draw & Label Enthalpy Diagrams - Video & Lesson ... An enthalpy diagram is a method used to keep track of the way energy moves during a reaction over a period of time. Learn how to draw and label enthalpy diagrams, the definition of an enthalpy ...
PDF Exothermic vs endothermic reaction graphs Given the reaction: A + B --> Ca) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for ...
Endothermic Reactions: Definition, Example, Diagram and ... Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.


























0 Response to "41 diagram of exothermic reaction"
Post a Comment