41 phase diagram for co2
The calculation of reliable phase diagrams of CO2 mixtures provides guidelines for optimization of compression, pipeline transport, and storage processes. Keywords: thermodynamic properties;thermophysics; transport properties, phase diagrams CO2 N2 mixutres,CO2 compression. Decomposes, without melting, into Na2CO3, H2O, and CO2 at 270 ºC. Density and other properties. 8 presents the phase diagram for glycols. There are other natural antifreeze substances; besides alcohols, sugars and salts, there are some antifreeze proteins that may keep cells alive down to -15 ºC.
Phase behavior plays an important role in a variety of enhanced oil recovery (EOR) processes. Such processes are designed to overcome, in one way or another, the capillary forces that act to trap oil during waterflooding.
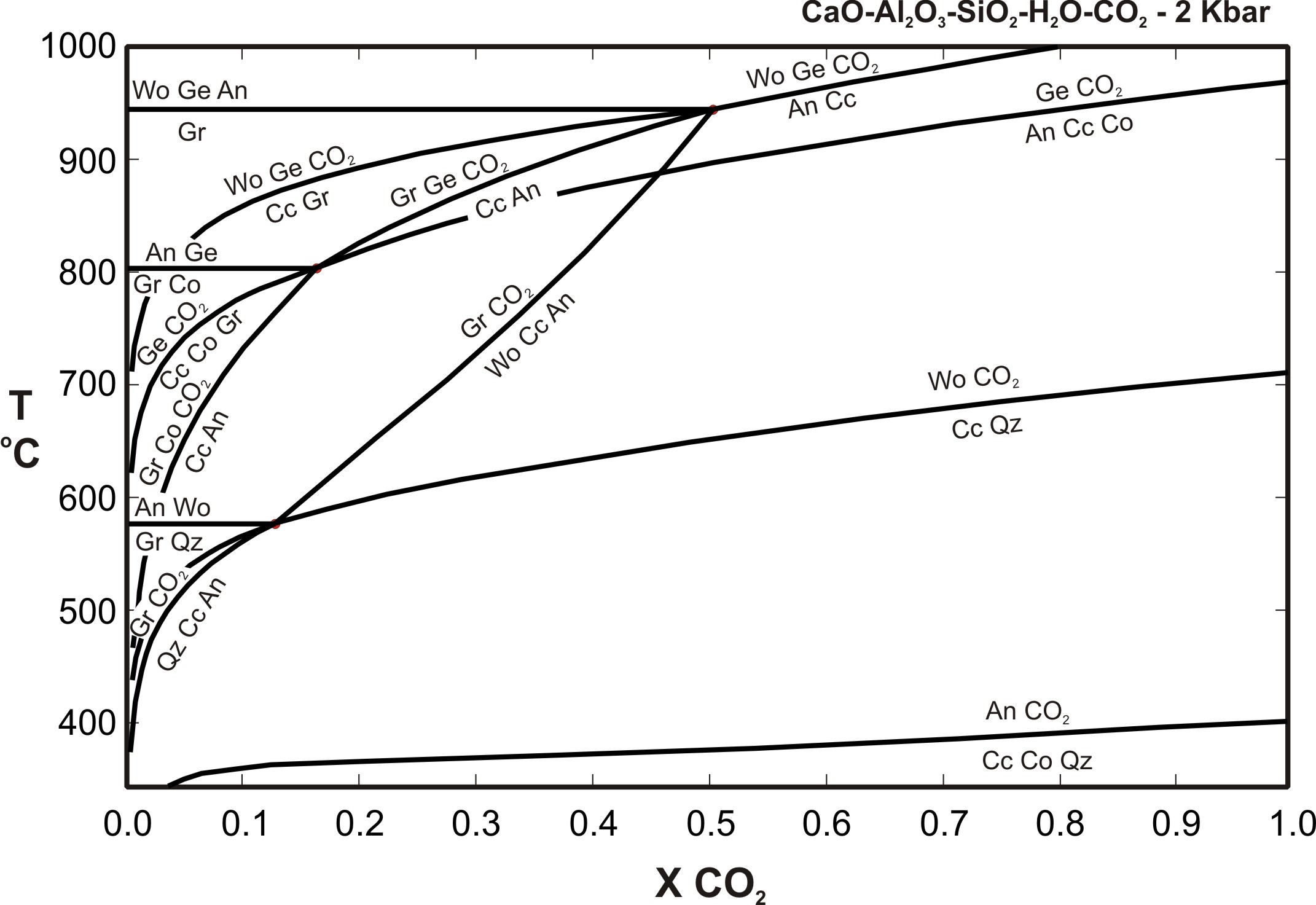
Phase diagram for co2
A phase diagram embodies infor-mation derived from the thermodynamic principles described in Chap. 2, specialized for a particular range of compositions The present chapter provides some fundamental concepts and tools for analyzing phase diagrams building upon the basics of thermodynam-ics and... Effect of t & composition (co). COMPONENTS, PHASES and Microconstituents. Reading phase diagrams. PHASE DIAGRAM - ex. PHASE DIAGRAMS: weight fractions of phases. The lever rule: a proof. COOLING OF Cu-Ni BINARY, equilibrium microstructure development. composition, showing relative quantities of phases at equilibrium • Pressure influences phase structure - Remains virtually constant in most applications - Most solid-state phase diagrams are at 1 atm. • Note: metastable phases do not appear on equilibrium phase diagrams.
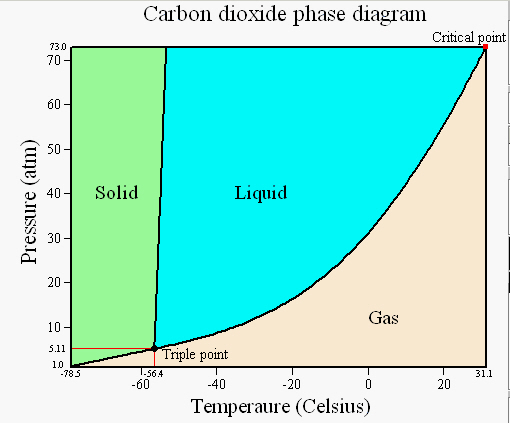
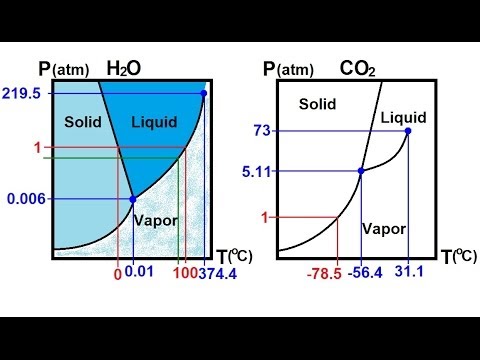
Phase diagram for co2. Phase Diagram of Carbon Dioxide Background When ice cream trucks drive through towns on hot summer days, how do you think they keep their The unique triple point for CO 2 and H2O also explains the differences in the phase diagrams. The triple point for: carbon dioxide is -57ºC at 5.2... Phase diagrams are used to map out the existence and conditions of various phases of a give system. The phase diagram of water is a common example. Solidication of alloy Co starts on cooing at T1.The rst solid formed has a composition of Cs1 and the liquid Co. On further cooling the solid... Phase Diagram - . is a graphical representation of the conditions of temperature and pressure at which solids, liquids. Phase Diagram for H2O. Vapor pressure Surface tension Viscosity Adhesive/cohesive forces Capillary action Density Compressibility Diffusion Evaporation The Liquid... Triple Point. Vapor. Copyright © 1999 ChemicaLogic Corporation Drawn with CO2Tab V1.0. Temperature, "C.
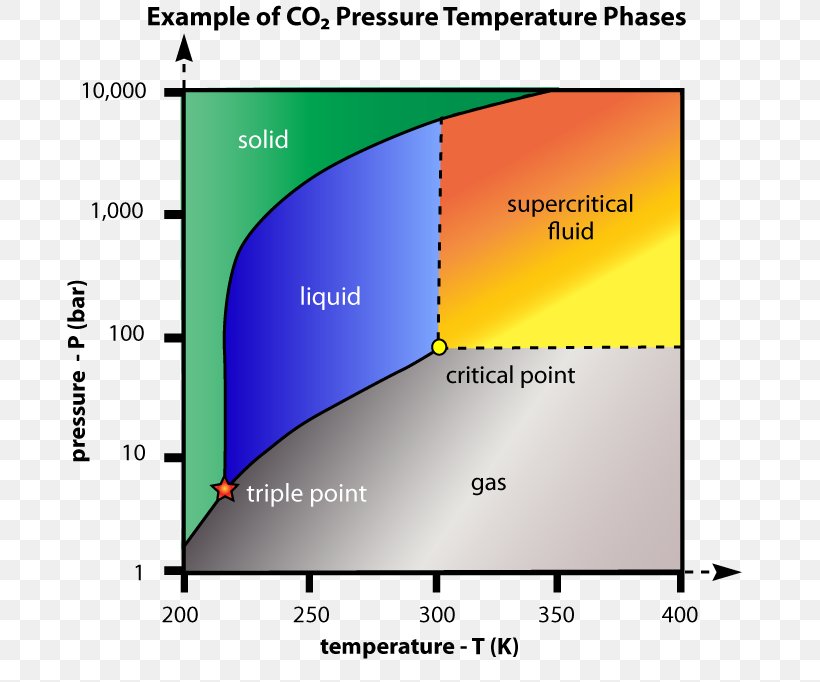
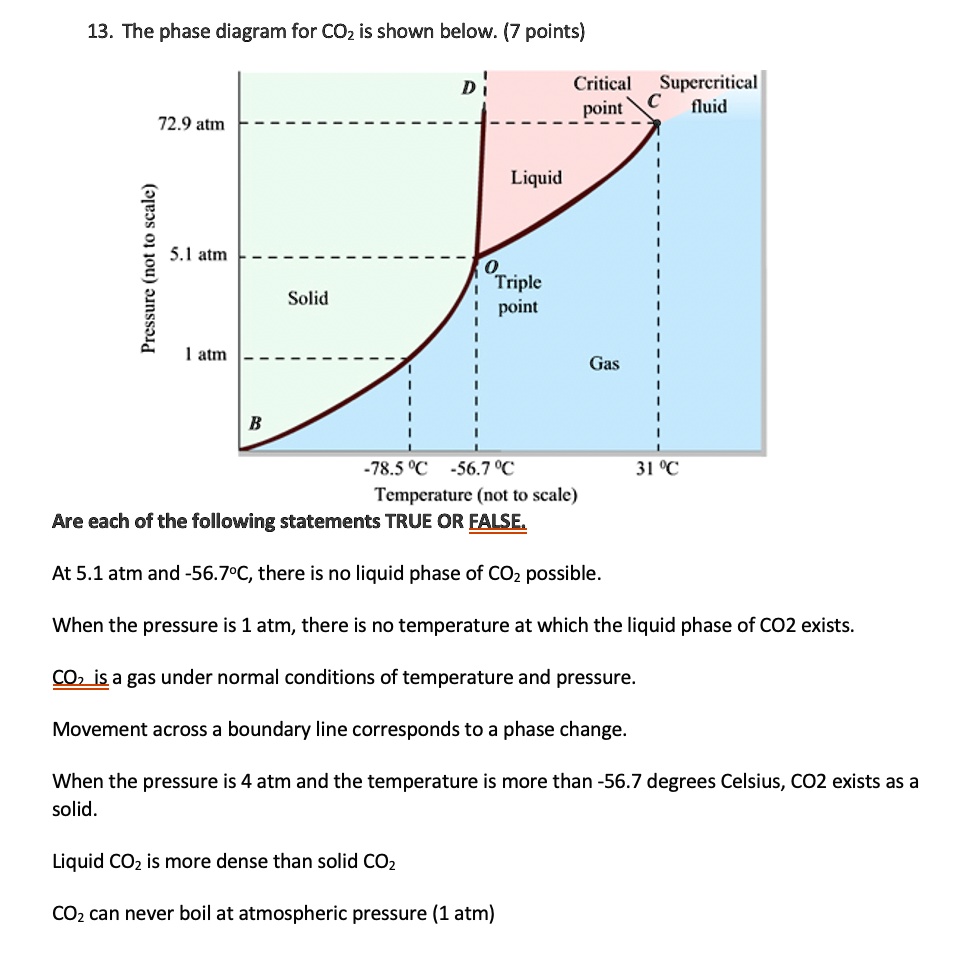
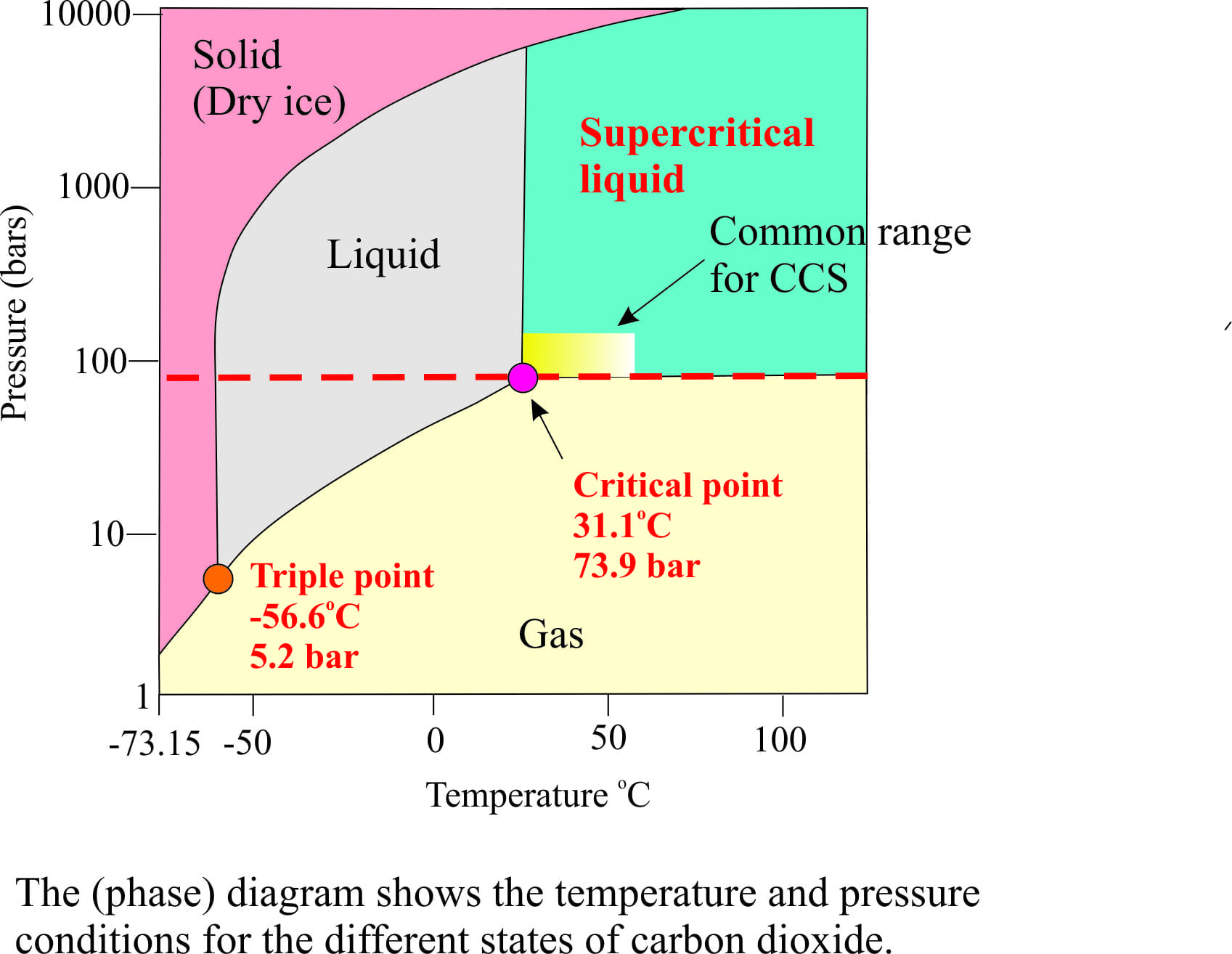
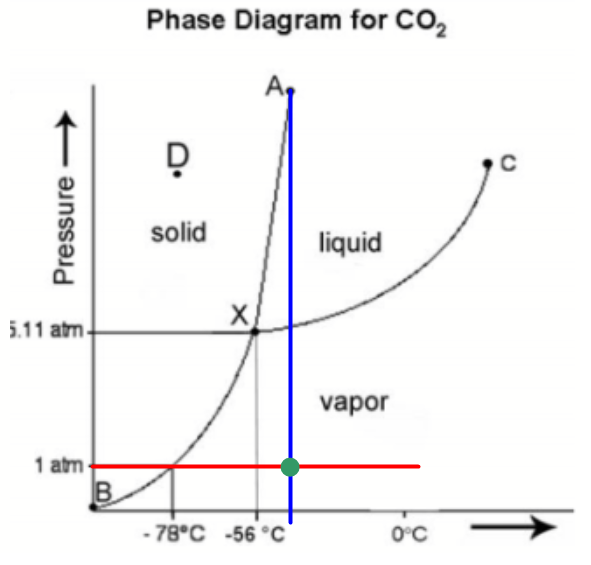
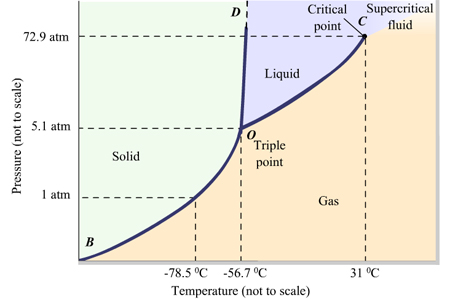
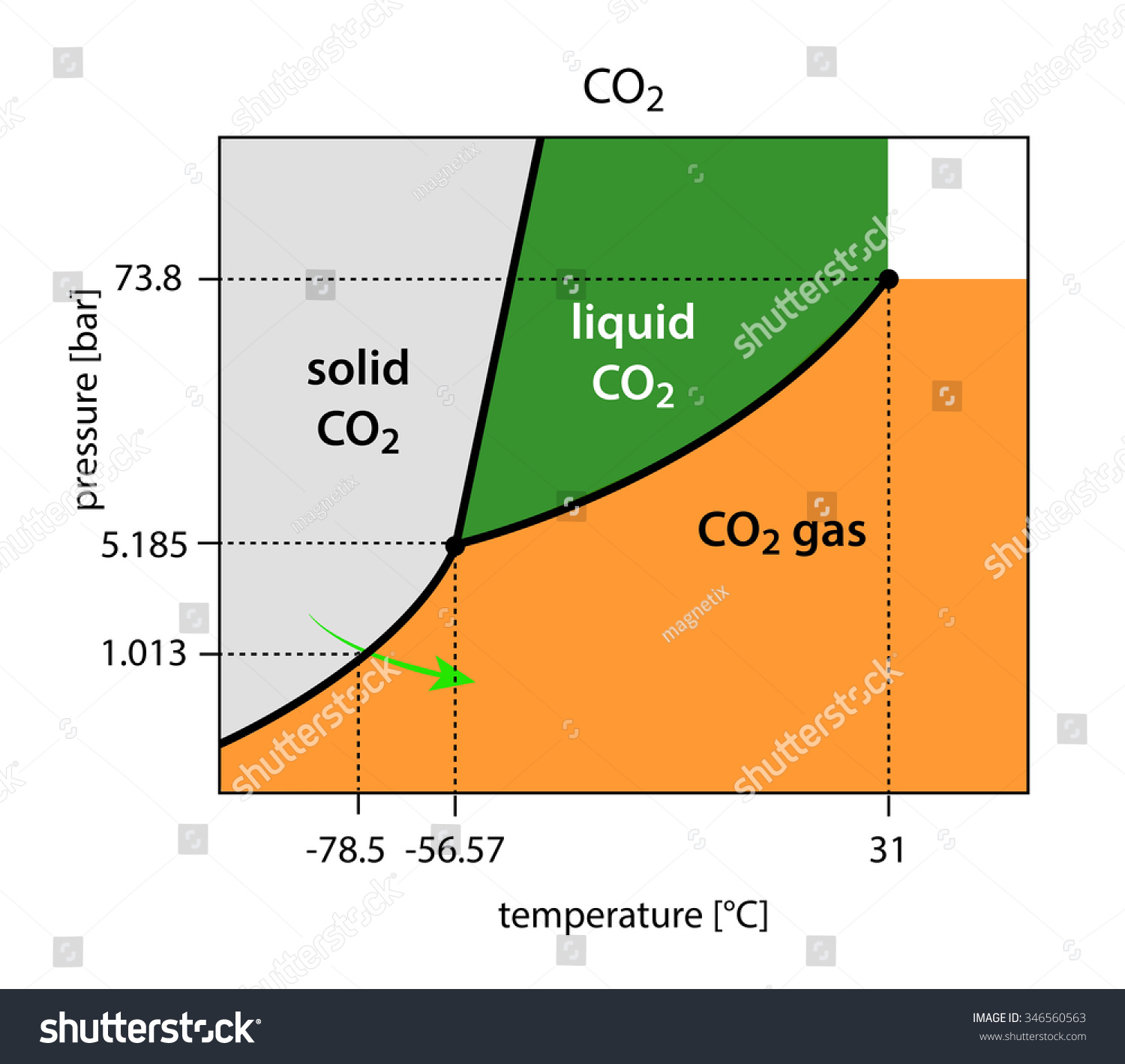
Given: phase diagram, temperature, and pressure. Asked for: physical form and physical changes. Identify the region of the phase diagram corresponding to the initial conditions and identify the Solid CO2 is generally known as dry ice because it is a cold solid with no liquid phase observed when it is... Figure-1: Unary phase diagram for water. Binary diagrams: These diagrams constitutes two components, e.g.: two metals (Cu and Ni), or a metal and a compound (Fe and Fe3C), or two These single-phases regions are separated by a two-phase region where both liquid and solid co-exist. Carbon dioxide, CO2, is a colourless and odorless gas. It is relatively nontoxic and noncombustible, but it is heavier than air and may asphyxiate by the displacement When CO2 is solved in water, the mild carbonic acid, is formed. Cooled CO2 in solid form is called dry ice. Carbon dioxide phase diagram. CO2 is a component of our atmosphere that is essential to life. It has no ozone depletion potential and insignificant global warming potential, so CO2 has no regulatory liability The supercritical portion of the transcritical cycle takes place above 1,067 psia. The phase diagram for CO2 is shown in Figure 1.
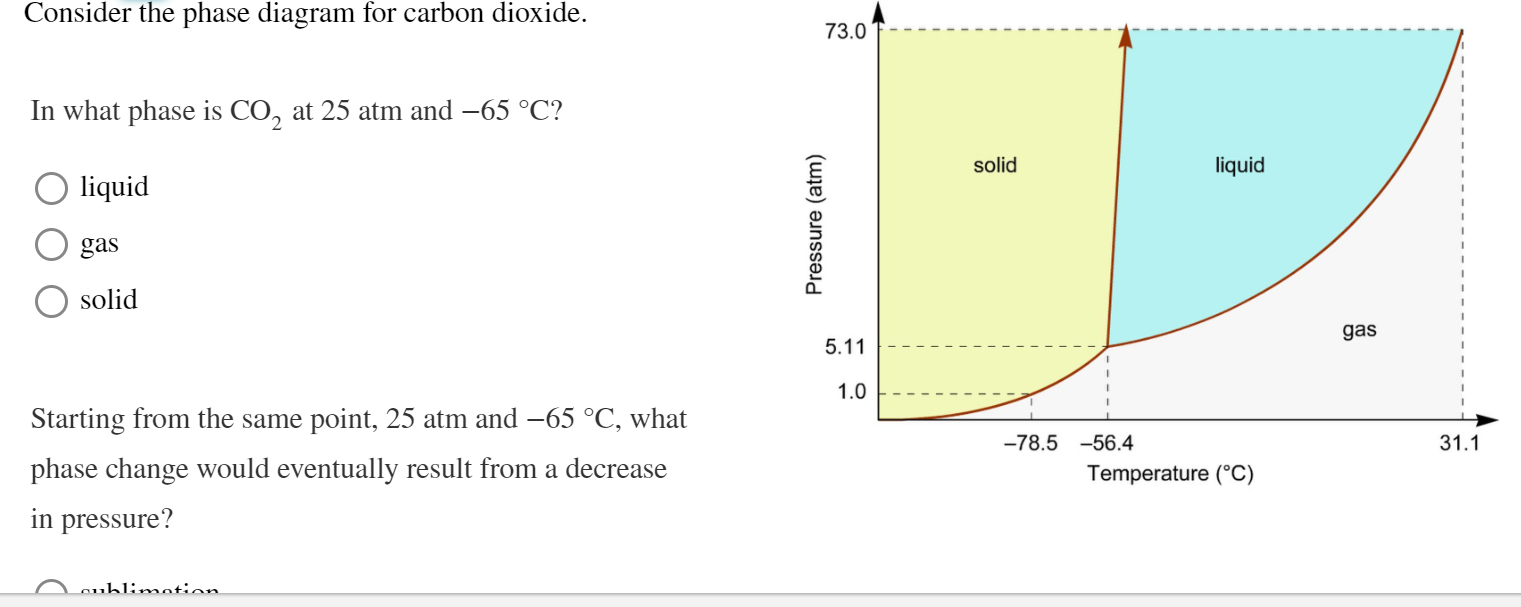
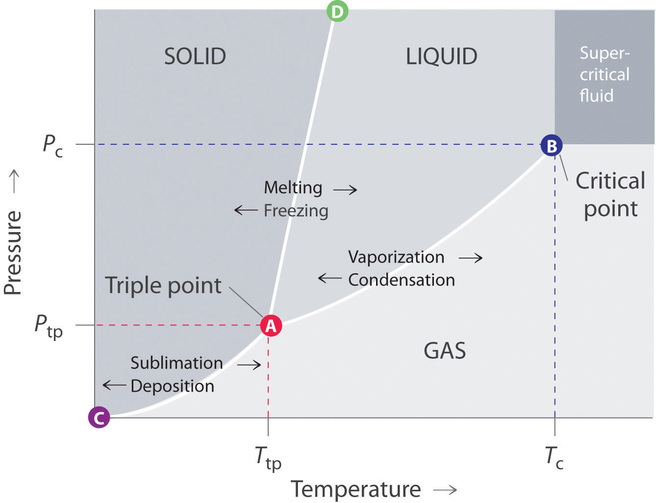
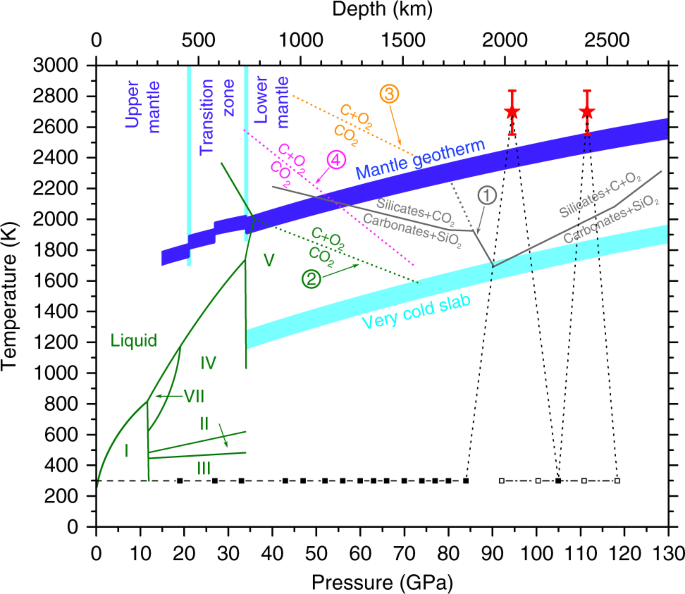
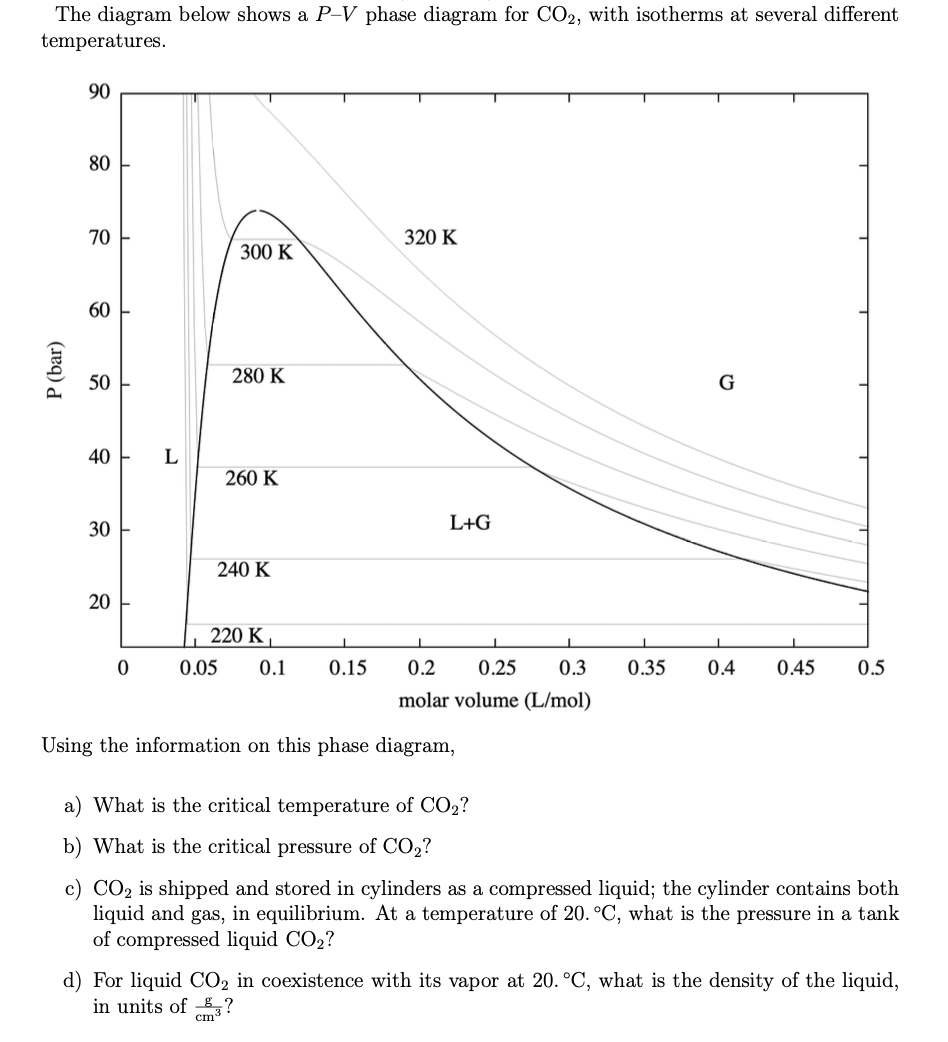
Some phase diagrams (those for 1-component diagrams) depict relationships involving multiple phases having the same composition (for example, the relationships between the vapor Metamorphic fluids are generally dominated by H2O and CO2, and the ratio H2O:CO2 can control mineral stability. Figure 2.10. Isothermal phase diagrams of the NaCl−Na2SO4− H2O, NaCl−Na2CO3− H2O, NaHCO3− Na2CO3− H2O, and NaCl−NaHCO3 The generalized phase diagram for the trivalent lanthanides is shown in Fig. 3A. This single diagram, which is also valid for yttrium-lanthanide binary... CO_2 is often transported In liquid form in pressurized tanks. Using the phase diagram, determine the minimum pressure required to keep CO_2 in the liquid phase at 20 degree C. Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO2 increases with pressure as it does for most substances (water being a notable exception as described previously).
Presentation on theme: "Phase Diagram for CO 2. Phase Diagram for H 2 O."— Presentation transcript 3 Phase Diagram for H 2 O. 4 The Liquid State Vapor pressure Surface tension Viscosity Adhesive/cohesive forces Capillary action Density Compressibility Diffusion Evaporation.
FactSage 8.1 - List of Stored Phase Diagrams (7811). Click on a system to display the phase diagram. Al-Co-O_Al2O3-CoO-O2_air FToxid. Al-Cr BINS.
A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. Determining the State of Carbon Dioxide Using the phase diagram for carbon dioxide shown in Figure 5, determine the state of CO2 at the following...
According to the phase diagram of carbon dioxide, under those conditions of temperature and pressure the solvent lies on the supercritical region [16]. ... It means that CO2 could diffuse into materials and be absorbed depending on the saturation conditions and the solubility of CO2 in the matrix.
9 hours ago The phase diagram of CO2 is similar to the phase diagram of water: sublimation curve, vaporization curve, triple point, critical temperature and Using the phase diagram for carbon dioxide shown in Figure 5, determine the state of CO 2 at the following temperatures and pressures: (a) −30 °...
Thermodynamic Properties of R744 (Carbon Dioxide - CO2). Critical Pressure: 7.377 MPa, Critical Temperature 30.978°C Ideal Gas Constant of Carbon Dioxide: R = 0.1889 kJ/kg.K. CO2 Property Tables
Pressure-temperature phase diagram of carbon dioxide. In particular, the use of renewable energy for production of fuels from CO2 (such as methanol) is attractive as this could result in fuels that could be easily transported and used within conventional combustion technologies but have no net CO2...
A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page These diagrams (including this one) are nearly always drawn highly distorted in order to see what is going on more easily. There are usually two...
composition, showing relative quantities of phases at equilibrium • Pressure influences phase structure - Remains virtually constant in most applications - Most solid-state phase diagrams are at 1 atm. • Note: metastable phases do not appear on equilibrium phase diagrams.
Effect of t & composition (co). COMPONENTS, PHASES and Microconstituents. Reading phase diagrams. PHASE DIAGRAM - ex. PHASE DIAGRAMS: weight fractions of phases. The lever rule: a proof. COOLING OF Cu-Ni BINARY, equilibrium microstructure development.
A phase diagram embodies infor-mation derived from the thermodynamic principles described in Chap. 2, specialized for a particular range of compositions The present chapter provides some fundamental concepts and tools for analyzing phase diagrams building upon the basics of thermodynam-ics and...





















0 Response to "41 phase diagram for co2"
Post a Comment