41 energy diagram organic chemistry
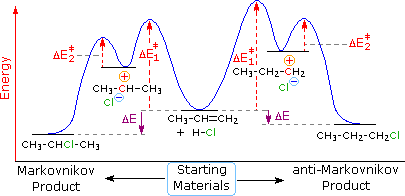
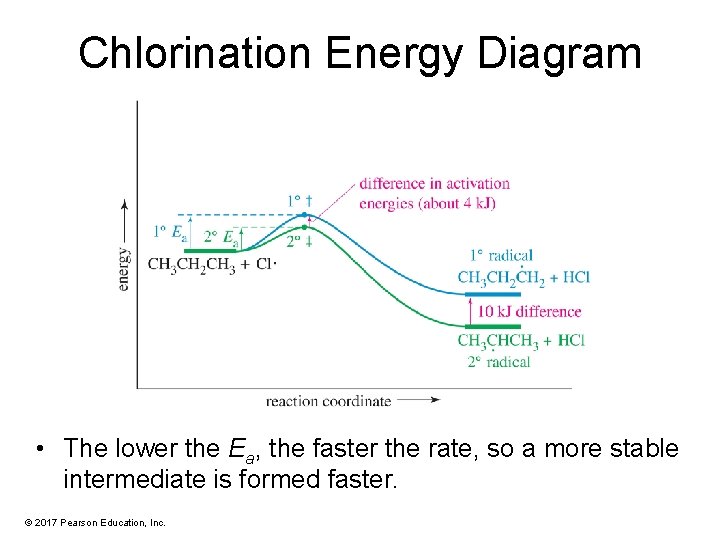
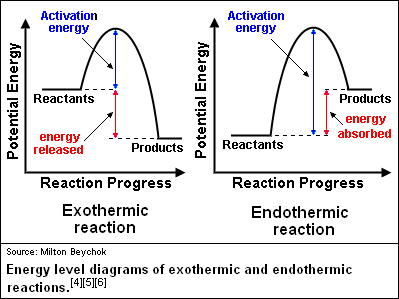
What is energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. The closest shell to the nucleus is called the “K shell” followed by the “L shell” then the “M shell” and so on away from the nucleus. The shells can be denoted by alphabets (K, L ... in the energy diagram)! Selectivity: if more than one site is available for reaction the ratio between each product obtained determines the selectivity! (the difference in E a for each competing path in the energy diagram)! This leads to an almost universal statement in organic chemistry:! FOR A GIVEN REACTION THE MORE REACTIVE, THE LESS SELECTIVE!
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
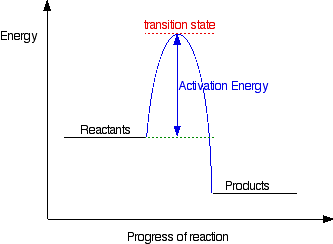
Energy diagram organic chemistry
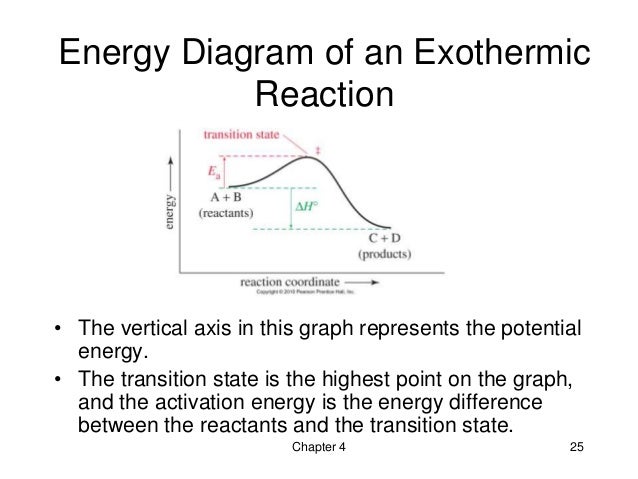
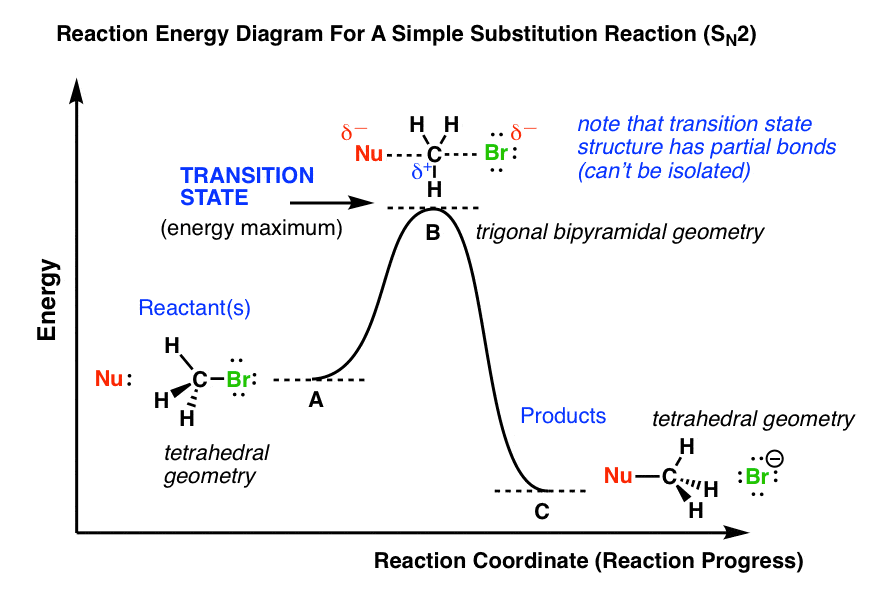
The potential energy diagram can illustrate the mechanism for a reaction by showing each elementary step of the reaction with distinct activation energy (see figure below). https://chem.libretexts.org/@a...y_with_a_Biological_Emphasis_(Soderberg)/Chapter_06%3A_Introduction_to_organic_reactivity_and_catalysis/6.2%3A_Energy_diagrams ... You may recall from general chemistry that it is often convenient to describe chemical reactions with energy ... crashcourseThermodynamics and Energy Diagrams: Crash Course Organic Chemistry #15. In organic chemistry, different reactions can take place at vastly different speeds. To better understand whether a reaction actually will happen, and how useful that reaction is, we need to understand thermodynamics and kinetics.
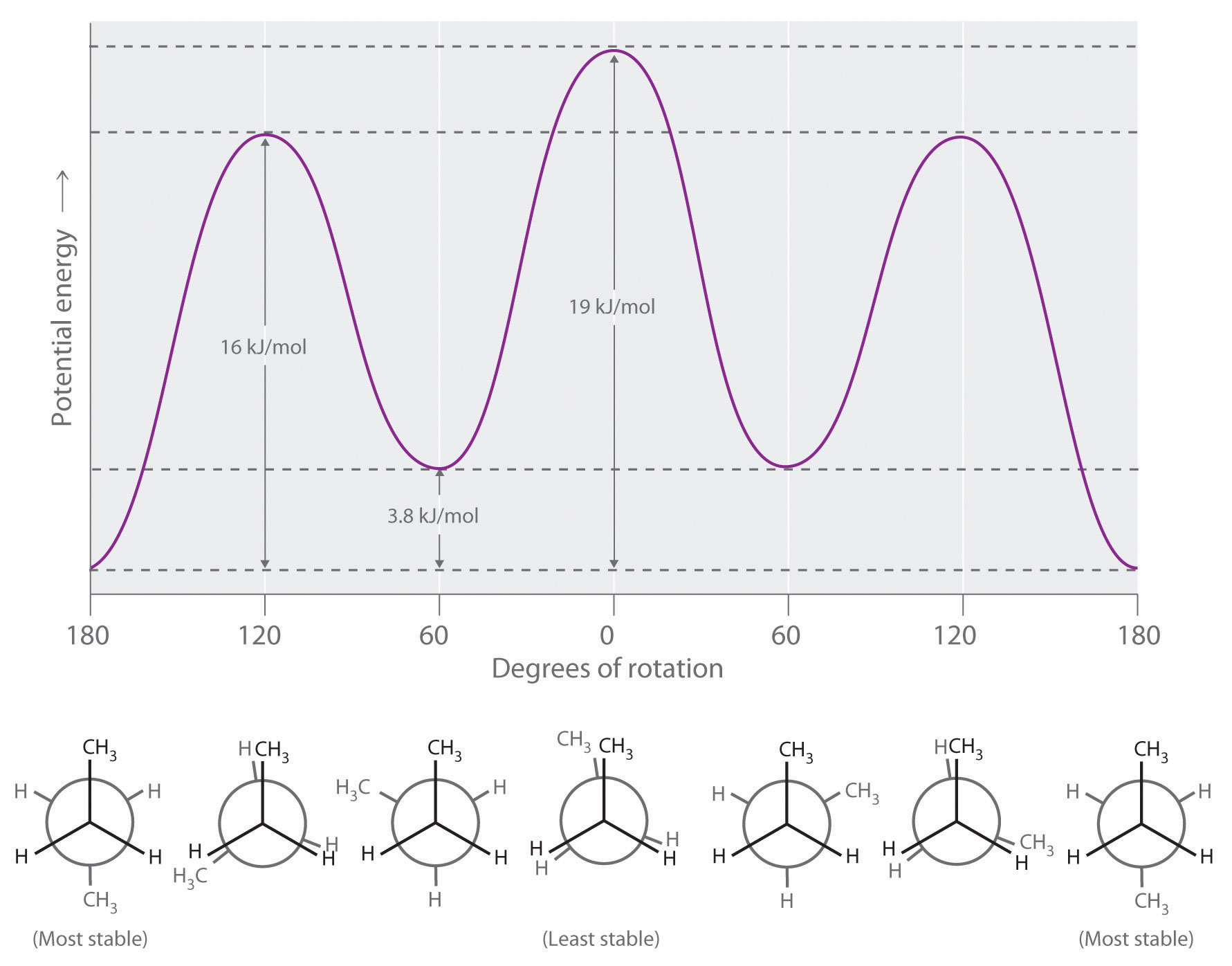
Energy diagram organic chemistry. It's time to learn a little more about a chemical reaction. How do molecules have to be arranged and how much energy do they have to collide with? What's a c... JoVE publishes peer-reviewed scientific video protocols to accelerate biological, medical, chemical and physical research. Watch our scientific video articles. I've been unable to find an energy diagram for propane either in my book or online via Google. So I resorted to making my own. I suspect that propane isn't covered because it's essentially the same deal as ethane - we can have one staggered conformation and one eclipsed conformation (only in propane it's a methyl eclipsing a hydrogen, not hydrogens eclipsing each other). If you're seeing this message, it means we're having trouble loading external resources on our website · If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked
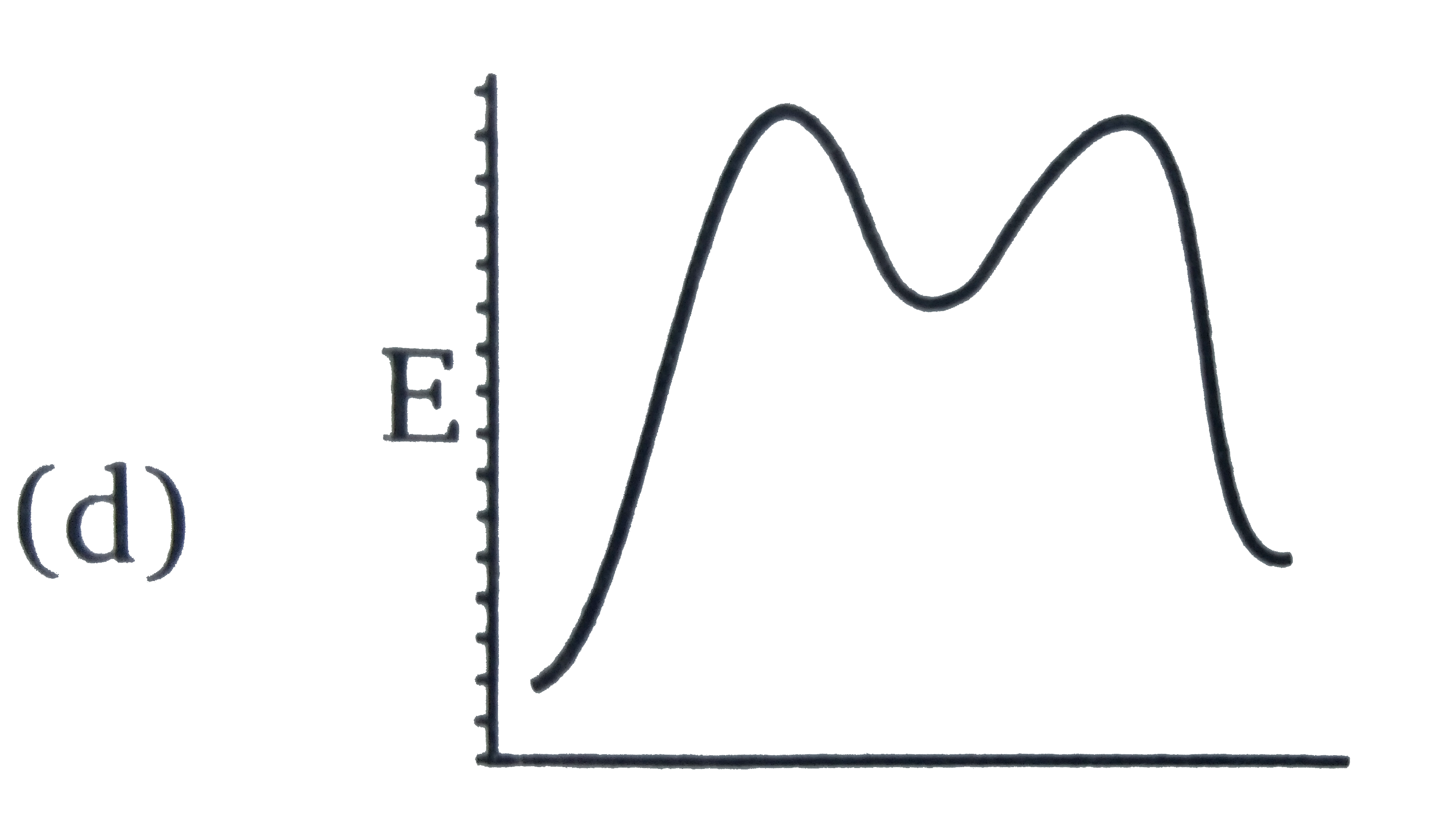
July 14, 2020 - Reaction energy diagrams efficiently and effectively communicate the thermodynamics and kinetics of chemical reactions in a single diagram. They are a useful tool in learning organic chemistry. In organic chemistry, different reactions can take place at vastly different speeds. To better understand whether a reaction actually will happen, and how us... Reaction coordinate diagrams are derived from the corresponding potential energy surface (PES), which are used in computational chemistry to model chemical reactions by relating the energy of a molecule(s) to its structure (within the Born–Oppenheimer approximation). Student Learning Outcomes After viewing this tutorial and practicing problems from this website and your textbook, you should be able to: • Identify the various parts of a reaction energy diagram and determine whether the reaction, as well as each steps of the reaction, is endothermic or ...
Arizona State University in Tempe, AZ is a public research university ranked #1 in the U.S. for innovation, dedicated to accessibility and academic excellence. Rotational Energy Profiles for H 3C–CH3 and (CH3)3C-CH3 Using the graph provided below, sketch the Rotational Energy Profile for ethane and neopen-tane. On the graph, also designate the minima (staggered conformations) and maxima (eclipsed conformations). –180–150–120 –90 –60 –30 0 30 60 90 120 150 180 0 2 4 6 8 10 12 14 16 18 ... Organic Chemistry/Introduction to reactions/Energy diagrams The kinetics has to do with the activation energy that it takes in order to make a reaction go forward. The activation energy in the free-energy diagram I gave you above would be the difference in the energy between the beginning and your highest point in the reaction. This would be my activation energy.
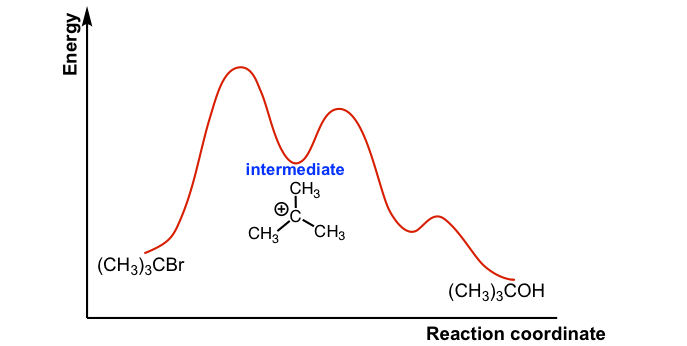
If a catalyst lowered the step one activation energy to a value lower than the step two activation energy, which step would be rate-limiting? ... Chemistry Concepts Intermediate. Authored by: Calbreath, Baxter, et al.. Provided by: CK12.org. Located at: http://www.ck12.org/book/CK-12-Chemistry-Co...
February 21, 2014 - This is part 1 of a four part series in the Energy Diagram Module. In order to talk about reaction mechanisms, we have to first understand what makes up a reaction. It may help to think of a reaction as a landscape. Let us look at the components in Graph 1 below. the x-axis as…
Video explaining Energy Diagram for Organic Chemistry. This is one of many videos provided by Clutch Prep to prepare you to succeed in your college classes.
You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ‘reaction coordinate’, tracing from left to right ...

Electrophilic Aromatic Substitution Energy Diagram Chemistry Organic Chemistry Organic Chemistry Notes
Reaction coordinate diagrams | Organic Chemistry 1: An open textbook. 5.3. Reaction coordinate diagrams. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ...
Draw a relative energy diagram showing a conformational analysis of 1,2-dichloroethane. Clearly label all staggered conformations and all eclipsed conformations with the corresponding Newman projections. Q. Sketch an approximate potential energy diagram for rotation about the carbon–carbon bond in 2,2-dimethylpropane similar to that shown in ...
crashcourseThermodynamics and Energy Diagrams: Crash Course Organic Chemistry #15. In organic chemistry, different reactions can take place at vastly different speeds. To better understand whether a reaction actually will happen, and how useful that reaction is, we need to understand thermodynamics and kinetics.
https://chem.libretexts.org/@a...y_with_a_Biological_Emphasis_(Soderberg)/Chapter_06%3A_Introduction_to_organic_reactivity_and_catalysis/6.2%3A_Energy_diagrams ... You may recall from general chemistry that it is often convenient to describe chemical reactions with energy ...
The potential energy diagram can illustrate the mechanism for a reaction by showing each elementary step of the reaction with distinct activation energy (see figure below).

Organic Chemistry Students Interpretations Of The Surface Features Of Reaction Coordinate Diagrams Semantic Scholar




















0 Response to "41 energy diagram organic chemistry"
Post a Comment