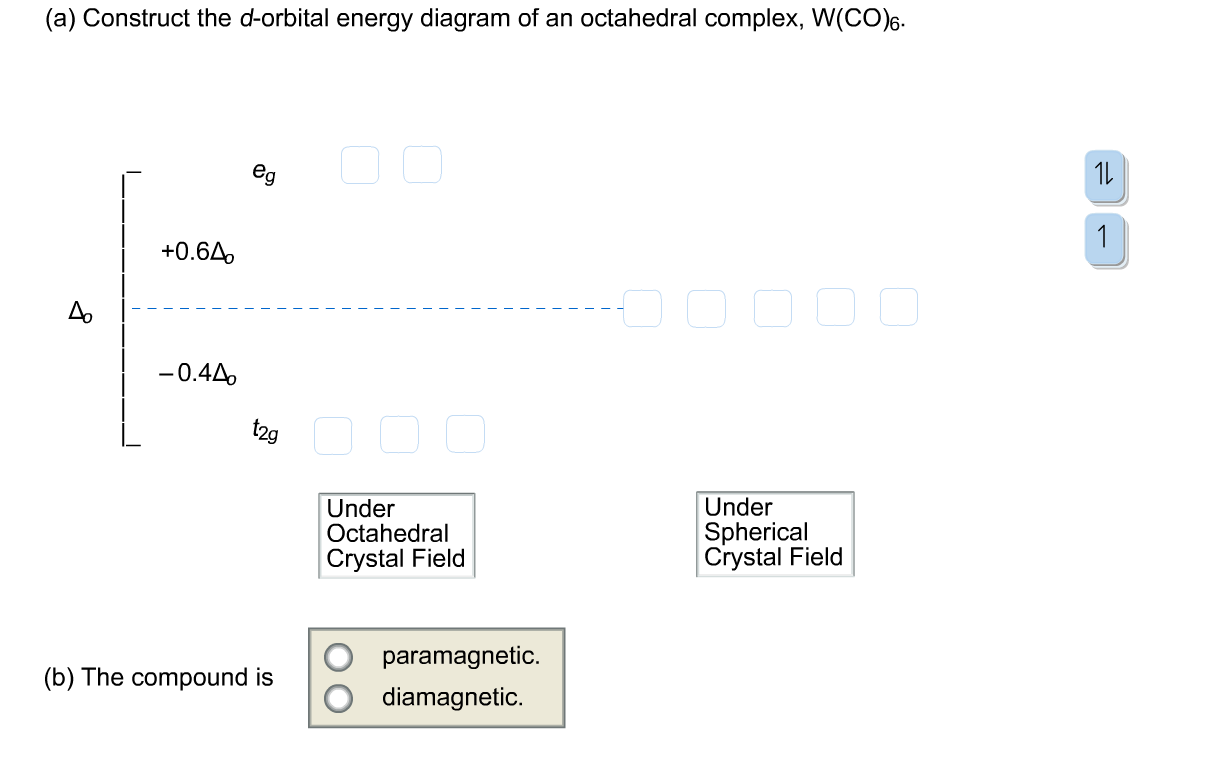
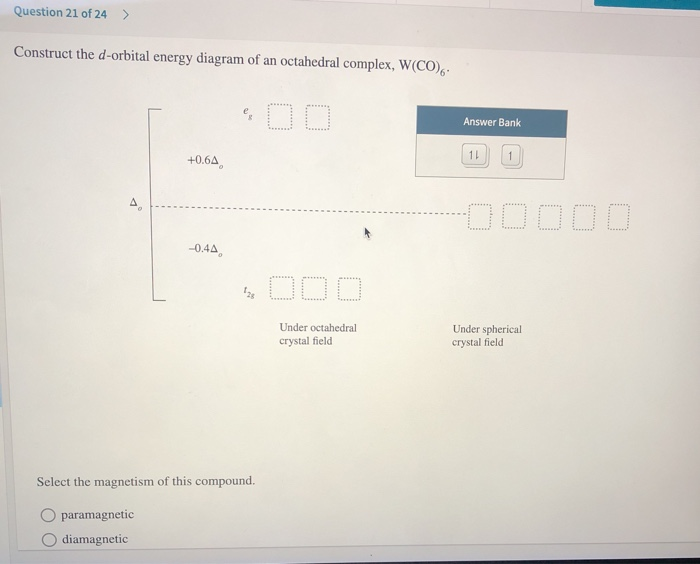
41 (a) construct the d-orbital energy diagram of an octahedral complex, w(co)6.
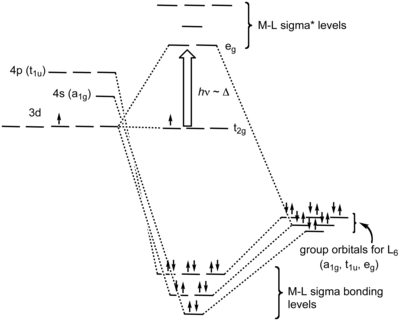
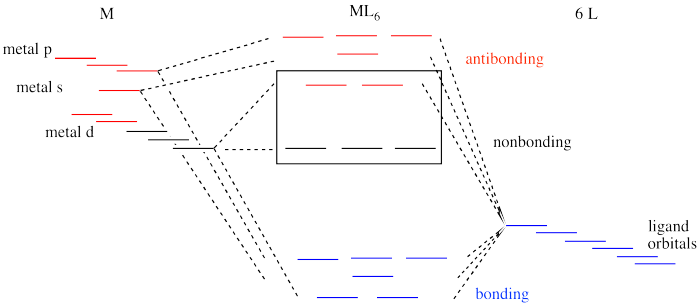
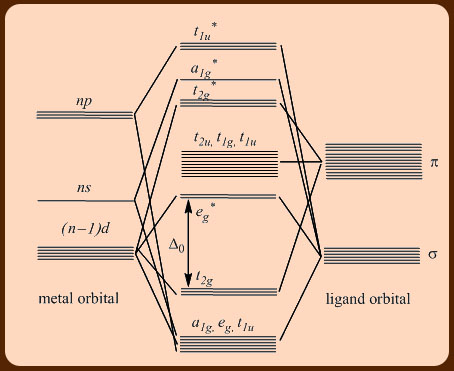
PDF Molecular Orbital Theory - Octahedral, Tetrahedral or ... The symmetry designations of different metal orbitals taking part in octahedral overlap are: dz2, dx2−y2- eg s - a1g px, py, pz- t1u dxy, dxz, dyz- t2g The exact nature of the Mulliken symbols, like egor a1g, etc., can be understood only after the core level understanding of group theory. Essentials of Physical Chemistry by B.S ... - Academia.edu Preface The Essentials of Physical Chemistry has been written for BSc students. It has been national bestseller for more than 65 years. It has been used by more than 2 million students. It is 26 editions old. It really has been that long. A lot of
YVES JEAN - Molecular Orbitals of Transition Metal ... Perturbation of the d orbitals: the general interaction diagram 107 Contents 3.2.4. A first example: the octahedral complex [ML5CO] 108 3.3. Complexes with several π -donor or π -acceptor ligands 111 3.3.1. The trans-[ML4Cl2] octahedral complex 111 3.3.2. The trans-[ML4(CO)2] octahedral complex 116 3.3.3.

(a) construct the d-orbital energy diagram of an octahedral complex, w(co)6.
Recent Progress in Emerging Two-Dimensional Transition ... 20/08/2021 · As a new member in two-dimensional materials family, transition metal carbides (TMCs) have many excellent properties, such as chemical stability, in-plane anisotropy, high conductivity and flexibility, and remarkable energy conversation efficiency, which predispose them for promising applications as transparent electrode, flexible electronics, broadband … Question: Construct the cf-orbital energy diagram of an ... Show transcribed image text Construct the cf-orbital energy diagram of an octahedral complex, W(CO)6. (b) The compound is Construct the cf-orbital energy diagram of an octahedral complex, W(CO)6. (b) The compound is order PDF Representations, Character Tables, and One Application of ... Character Tables List of the complete set of irreducible representations (rows) and symmetry classes (columns) of a point group. C2h EC2 i σh linear quadratic Ag 11 1 1R z x2, y 2, z , xy Bg 1-1 1 -1R x, R y xz, yz Au 1 1 -1 -1 z Bu 1-1 -1 1x, y irreducible representations
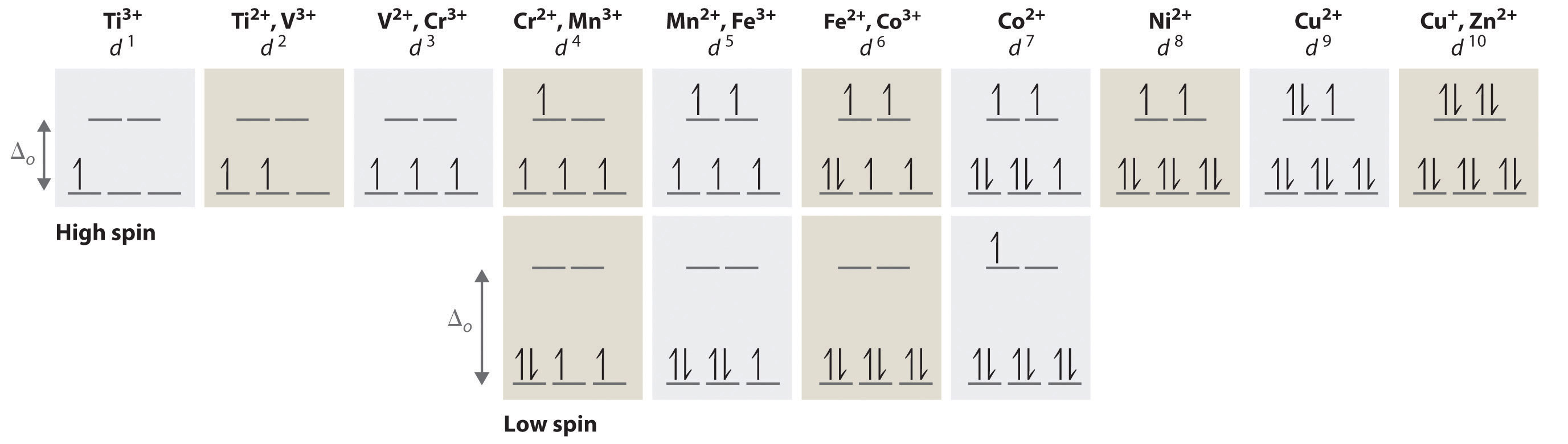
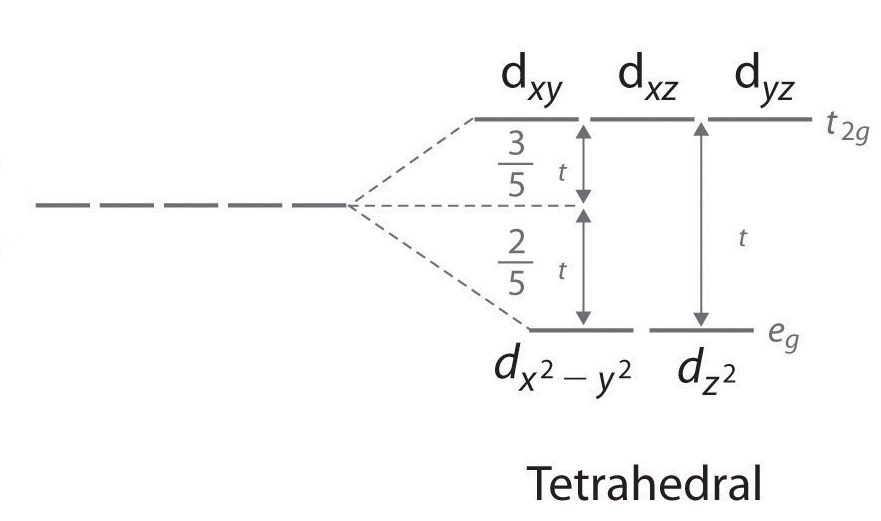
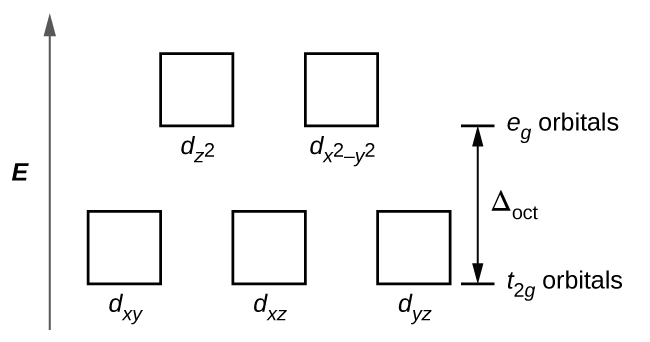
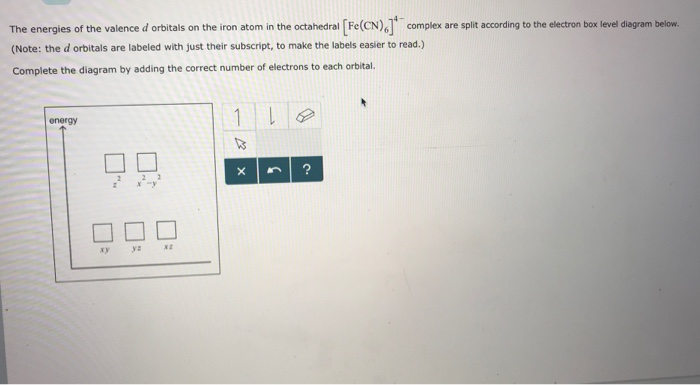
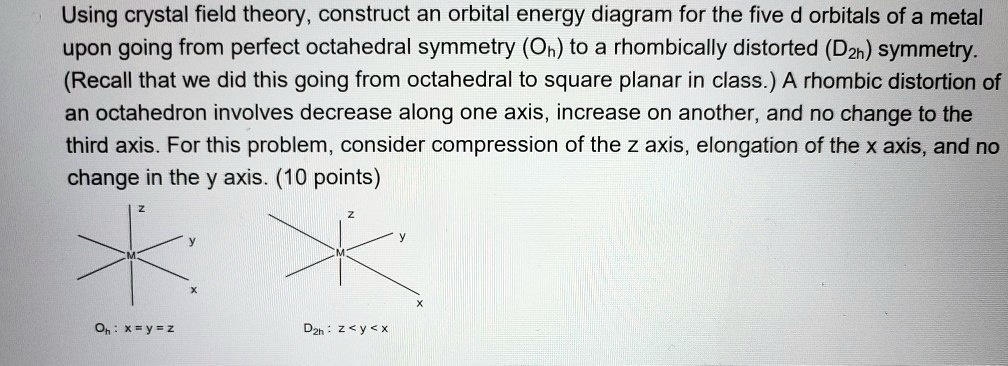
(a) construct the d-orbital energy diagram of an octahedral complex, w(co)6.. 8.2 Hybrid Atomic Orbitals - Chemistry Other atoms that exhibit sp 3 d 2 hybridization include the phosphorus atom in PCl 6 −, the iodine atom in the interhalogens IF 6 +, IF 5, ICl 4 −, IF 4 − and the xenon atom in XeF 4. Figure 15. (a) Sulfur hexafluoride, SF 6, has an octahedral structure that requires sp 3 d 2 hybridization. (b) The six sp 3 d 2 orbitals form an octahedral ... 4.3: High Spin and Low Spin Complexes - Chemistry LibreTexts In an octahedral complex, when Δ is large (strong field ligand), the electrons will first fill the lower energy d orbitals before any electrons are placed on the higher energy d orbitals. It is then classified as low spin because there is a minimal amount of unpaired electrons. Jahn-Teller Distortions - Chemistry LibreTexts For a given octahedral complex, the five d atomic orbitals are split into two degenerate sets when constructing a molecular orbital diagram. These are represented by the sets' symmetry labels: t 2 g ( d x z, d y z, d x y) and e g ( d z 2 and d x 2 − y 2 ). 19.3 Spectroscopic and Magnetic Properties of Coordination ... The five d orbitals consist of lobe-shaped regions and are arranged in space, as shown in Figure 1. In an octahedral complex, the six ligands coordinate along the axes. Figure 1. The directional characteristics of the five d orbitals are shown here. The shaded portions indicate the phase of the orbitals. The ligands (L) coordinate along the axes.
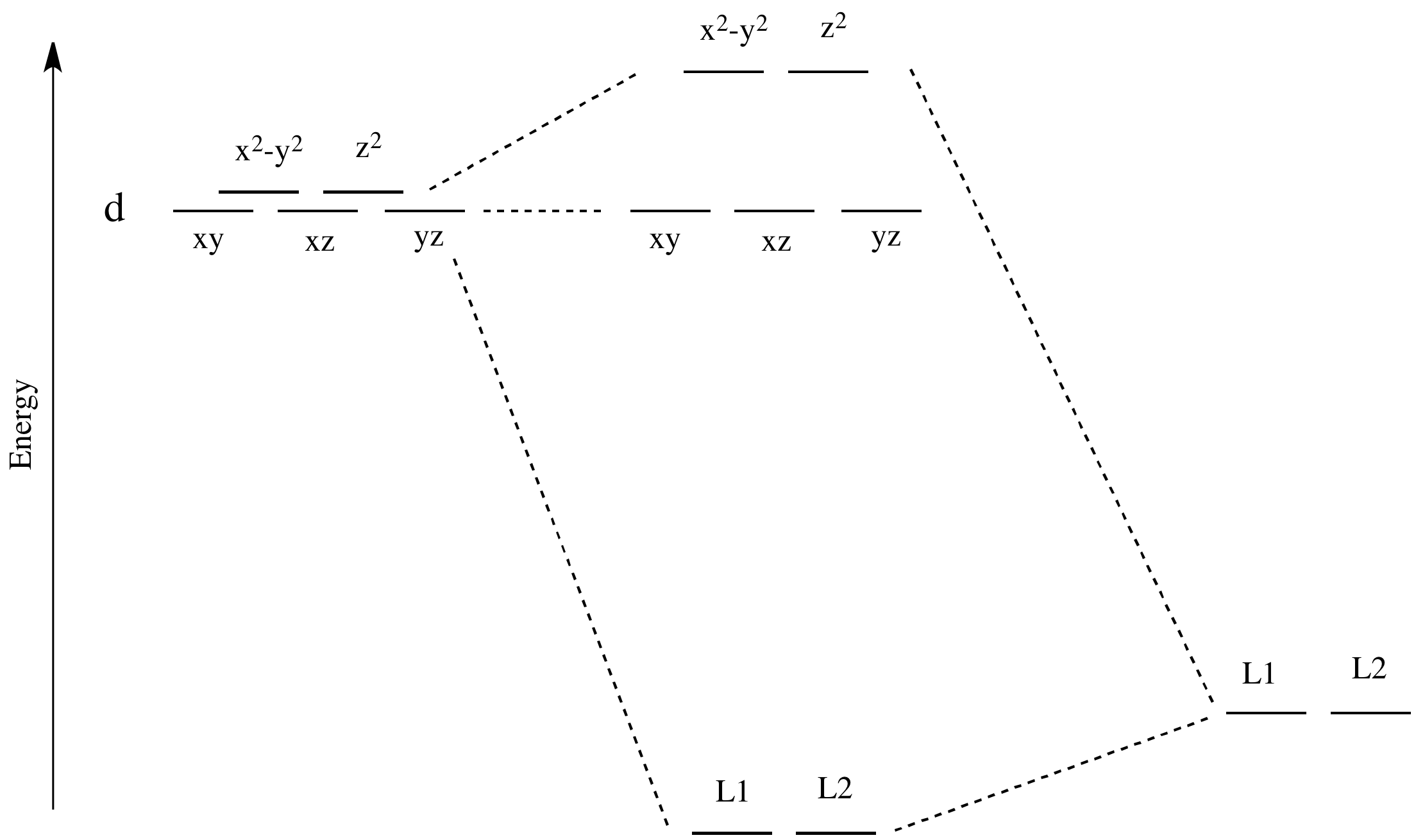
What is the correct molecular orbital diagram for the d ... I am trying to construct the diagram of $\mathrm{d}$ orbitals in the tetraammineplatinum(II) complex. According to the angular overlap model, $\mathrm{d}$ orbitals are going to look like those in the picture. However, planar square complexes are drawn completely differently according to other approaches and this makes me doubt the validity of my diagram. PDF Chem 59-250 - University of Delaware d orbital functions can also be treated in a similar way E C 2 σ v (xz) σ ' v (yz) No change ∴ symmetric ∴ 1's in table y x Symmetry of orbitals and functions The z axis is pointing out of the screen! So these are representations of the view of the d z 2 orbital and d x 2-y 2 orbital down the z-axis. y x y x Solved Construct the d-orbital energy diagram of an - Chegg Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 85% (13 ratings) Transcribed image text: Construct the d-orbital energy diagram of an octahedral complex, W (CO). Answer Bank +0.64, -0.44 WOOD Under octahedral crystal field Under spherical crystal field. PDF Magnetic Properties of Coordination Complexes Magnetic Properties of Coordination Complexes μ eff = 2√S(S+1) = √n(n+2) BM If there is a possibility for contribution from the orbital angular momentum, μ= √L(L+1) + 4S(S+1) For a given value of the orbital quantum number l, the magnetic quantum number m can have any values from -l to +l and L = sum of m For d orbital electrons,orbital electrons, m = 2, 1, 0,2, 1, 0, -1, -2
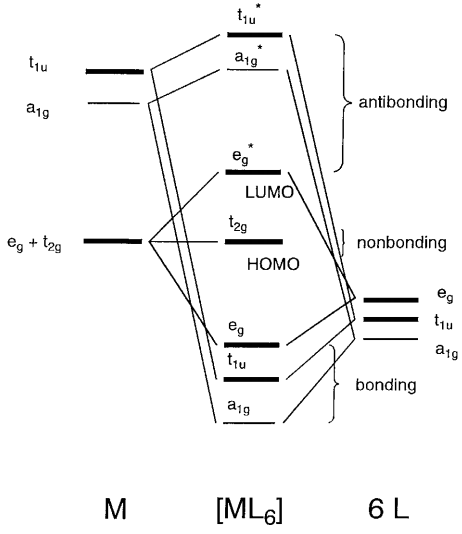
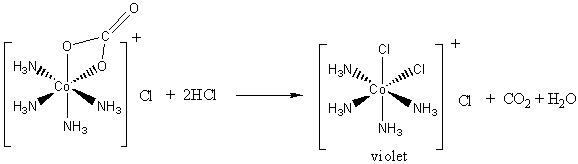
DOC Molecular Orbital Theory of Octahedral Complexes Molecular Orbital Theory of Octahedral Complexes Molecular Orbital Theory of Octahedral Complexes In contrast to crystal field theory, molecular orbital included the covalent nature of the metal-ligand bond interaction. No Metal- Ligand -bonding ( bonding only) Let's take [Co(NH3)6]3+ as an example. Nano Letters | Vol 22, No 3 09/02/2022 · A laser ablation method was deployed to prepare a series of earth-abundant transition metal selenide nanocrystals, among which Cu7.2Se4 with optimized scaling relationship between oxygenated intermediates was identified to efficiently drive the O2-to-H2O2 conversion at ambient condition. View the article. Self Test Answers - VSIP.INFO (a) Which orbitals will be displaced from their position in the octahedral molecular orbital diagram by π interactions with the lone pairs of the Cl- ligand? (b) Which orbital will move because the Cl- ligand is not as strong a base as NH3? (c) Sketch the qualitative molecular orbital diagram for the C4v complex. d-metal complexes Practice Problems Answers The hint concerning the color of [Co(en) 2 Cl 2] + is useful because the enantiomerically resolvable form of this ion must be the cis isomer (the trans isomer is not optically active). This suggests that the violet salt, CoCl 3 ·4NH 3, must have a cis structure, as well, shown below.. The air oxidation of CoCO 3 gives the pink cis-chelate complex:. Addition of HCl causes an acid/base reaction ...
Solved (a) Construct the d-orbital energy diagram of - Chegg Transcribed image text: (a) Construct the d-orbital energy diagram of octahedral complex [CoClol (b) How many unpaired electrons are there in [CoCl6l? Select answer 2g (c) Calculate the Ligand Field Stabilization Energy (LFSE) of this octahedral complex with respect to Ao Hint: consider the energy of electrons if placed in the following diagram ...
High-K materials and metal gates for CMOS ... - ScienceDirect 01/02/2015 · In all these transition metal oxides, the conduction band minimum at Γ lies at the metal d energy, while the top of the valence band are non-bonding O 2p states lying at the O 2p energy. Thus the band gap is a pure ionic gap between O 2p and metal d states. The band gap is proportional to the metal atomic d orbital energy .
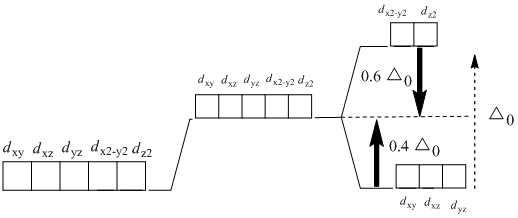
PDF Crystal Field Splitting in an Octahedral Field Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount of 0.6 Δo The t2gorbitals to be stabilized to the ...
Construct the d-orbital energy diagram of octahedral complex ... Given below is the molecular orbital diagram for octahedral complex V (HOP electrons for this complex. Fill orbitals w 19 3d — + b - + e. + aa Orbitals on metal Molecular orbitals Ligand group orbitals How many unpaired electrons are in the high-spin and low-spin states of Ru2+ in an octahedral...
Solved a) Construct the a-orbltal energy diagram of an ... Question: a) Construct the a-orbltal energy diagram of an octahedral complex, W (CO)6 +0.6A -0.4Ao Under Octahedral Crystal Field Under Spherical Crystal Field O paranagnetic O diamagnetic. (b) The compound is Previous & Give Up & View Solution Check Answer e Next This problem has been solved! See the answer Show transcribed image text
19.2 Coordination Chemistry of Transition Metals The chloride and nitrate anions in [Co(H 2 O) 6]Cl 2 and [Cr(en) 3](NO 3) 3, and the potassium cations in K 2 [PtCl 6], are outside the brackets and are not bonded to the metal ion. Figure 8. Many transition metal complexes adopt octahedral geometries, with six donor atoms forming bond angles of 90° about the central atom with adjacent ligands.
Solved Question 21 of 24 > Construct the d-orbital energy ... Question 21 of 24 > Construct the d-orbital energy diagram of an octahedral complex, W(CO), Answer Bank +0.64 00000 -0.44 Under octahedral crystal field Under spherical crystal field Select the magnetism of this compound. O paramagnetic O diamagnetic
Crystal Field Theory - Chemistry LibreTexts Placing a charge of −1 at each vertex of an octahedron causes the d orbitals to split into two groups with different energies: the d x2−y2 and d z2 orbitals increase in energy, while the, d xy, d xz, and d yz orbitals decrease in energy. The average energy of the five d orbitals is the same as for a spherical distribution of a −6 charge, however.
PDF Coordination Chemistry II: Ligand Field Theory Continued Electron Pairing Energy The total electron pairing energy, Π total, has two components, Πcand Πe •Πcis a destabilizing energy for the Coulombicrepulsion associated with putting two electrons into the same orbital •Πeis a stabilizing energy for electron exchange associated with two degenerate electrons having parallel spin total 3 e 0 c eg* t2g
Crystal Field Theory (CFT) - Detailed Explanation with ... The d-orbitals are fivefold degenerate in a free gaseous metal ion. If a spherically symmetric field of negative ligand filed charge is imposed on a central metal ion, the d-orbitals will remain degenerate but followed by some changes in the energy of free ion. A summary of the interactions is given below. Crystal Field Splitting
Solved Construct the d-orbital energy diagram of an ... Construct the d-orbital energy diagram of an octahedral complex, W(CO) 00 Answer Bank 11 +0.64 -0.44 ODO Under octahedral crystal field Under spherical crystal field Select the magnetism of this compound. O paramagnetic diamagnetic Which complex has the greatest A.? O [Fe(en)12+ O [Fe(OH),14- O [FeBr 14- O [Fe(H,0)612+
PDF ORBITALS and MOLECULAR REPRESENTATION In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ...
1.02: D-orbitals Splitting - Chemistry LibreTexts Mar 18, 2020 · D In a high-spin octahedral d 6 complex, the first five electrons are placed individually in each of the d orbitals with their spins parallel, and the sixth electron is paired in one of the t 2g orbitals, giving four unpaired electrons. A This complex has four ligands, so it is either square planar or tetrahedral.
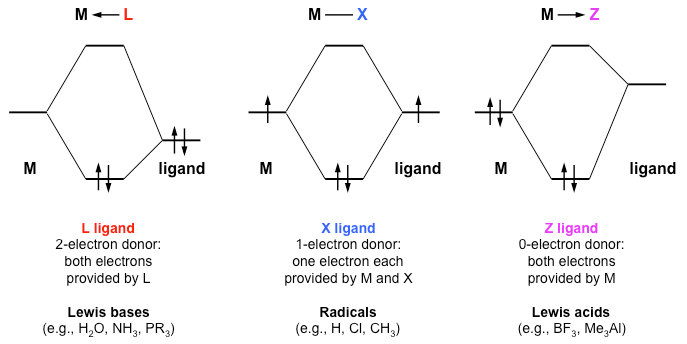
PDF Metal-Ligand and Metal-Metal Bonding Core Module 4 RED Recap of molecular orbital theory. s-donor ligands (hydride complexes). Construction and interpretation of octahedral ML 6 molecular orbital energy diagram Lecture 3: p-acceptor ligands, synergic bonding, CO, CN-, N 2, Lecture 4: Alkenes and alkynes. Dewar-Duncanson-Chatt model. Lecture 5: M(H 2) vs M(H) 2, M n(O 2) complexes, O 2, NO, PR 3 ...
PDF Representations, Character Tables, and One Application of ... Character Tables List of the complete set of irreducible representations (rows) and symmetry classes (columns) of a point group. C2h EC2 i σh linear quadratic Ag 11 1 1R z x2, y 2, z , xy Bg 1-1 1 -1R x, R y xz, yz Au 1 1 -1 -1 z Bu 1-1 -1 1x, y irreducible representations
Question: Construct the cf-orbital energy diagram of an ... Show transcribed image text Construct the cf-orbital energy diagram of an octahedral complex, W(CO)6. (b) The compound is Construct the cf-orbital energy diagram of an octahedral complex, W(CO)6. (b) The compound is order
Recent Progress in Emerging Two-Dimensional Transition ... 20/08/2021 · As a new member in two-dimensional materials family, transition metal carbides (TMCs) have many excellent properties, such as chemical stability, in-plane anisotropy, high conductivity and flexibility, and remarkable energy conversation efficiency, which predispose them for promising applications as transparent electrode, flexible electronics, broadband …










![d-orbital energy levels in planar [M II F 4 ] 2− , [M II (NH ...](https://pubs.rsc.org/image/article/2020/DT/d0dt02022b/d0dt02022b-f1_hi-res.gif)






![d orbital energies for an octahedral [MA 6 ] and tetragonal ...](https://www.researchgate.net/profile/Mihail-Atanasov/publication/262728830/figure/fig1/AS:925857749147649@1597753097674/d-orbital-energies-for-an-octahedral-MA-6-and-tetragonal-trans-MA-4-B-2-complexes.jpg)
0 Response to "41 (a) construct the d-orbital energy diagram of an octahedral complex, w(co)6."
Post a Comment