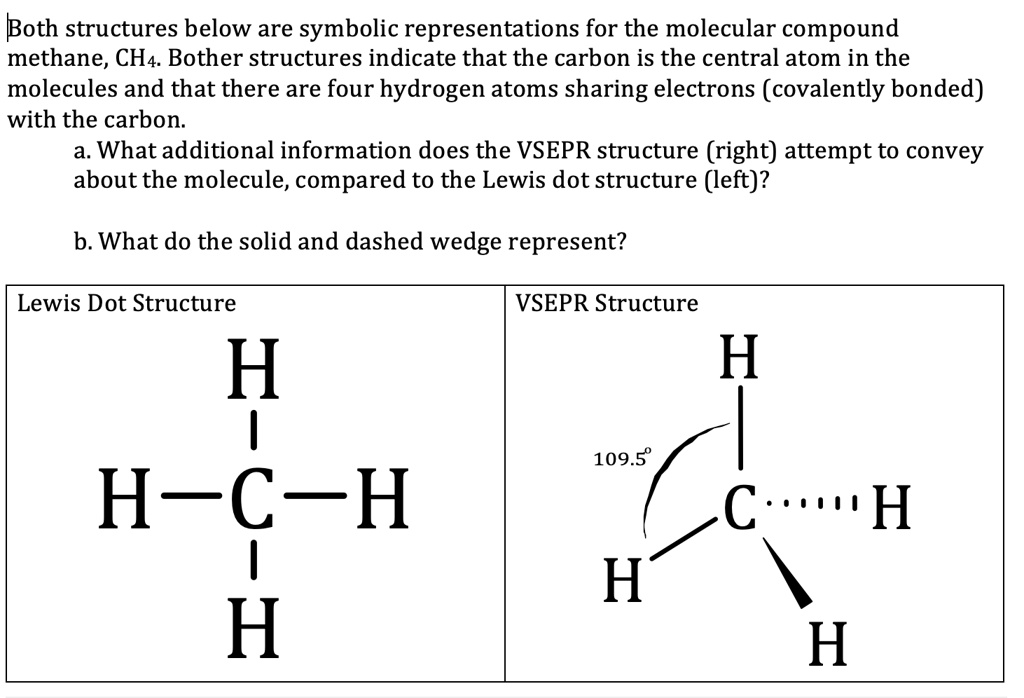
38 lewis diagram for methane
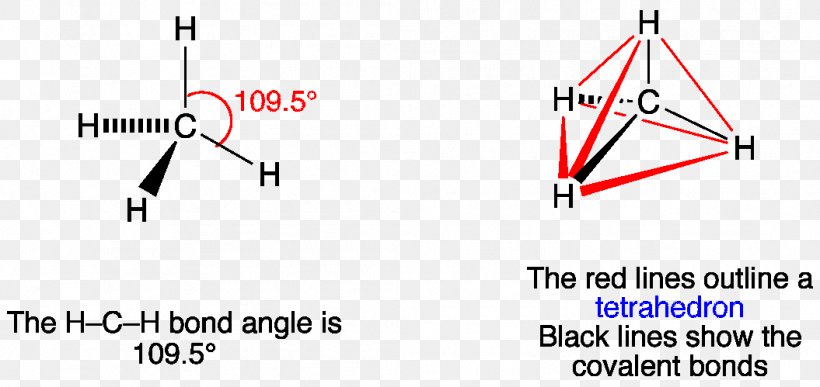
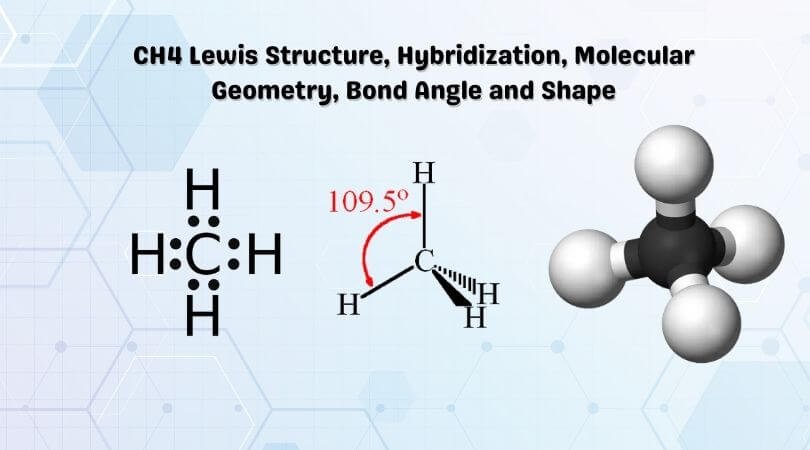
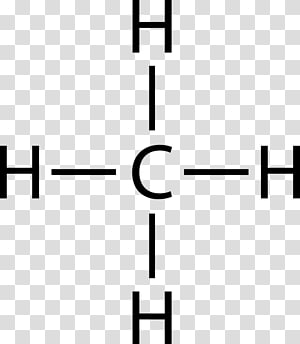
What is the Lewis dot structure of methane? - Answers Lewis Structure of Methane (CH4)H|H--C--H|HorH..H : C : H..H. Does CaCO3 have a Lewis structure? No, not exactly. It is an ionic compound so it would not have a Lewis dot structure. Lewis Structure of CH4 (Methane), Shape & Hybridization Feb 27, 2022 · Lewis structure of methane shows that the central atom C has four bonding electron pairs. These electron pairs repel each other and are thus directed to the four corners of a regular tetrahedron. A regular tetrahedron is a solid figure with four faces which are equilateral triangles. All bond angles are 109.5 °.
Lewis Dot of Methane CH4 - Kentchemistry.com Lewis Dot of Methane. CH 4. Back. 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). Methane is the first member of the alkane series.

Lewis diagram for methane
Methane | CH4 - PubChem Methane | CH4 | CID 297 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards ... CH4 Lewis Structure - How to Draw the Dot Structure for ... How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use... Methane: Molecular Geometry - Hybridization - Molecular ... Lewis structure: A Lewis structure or Lewis representation (also known as electron raster diagram, Lewis raster formula, Lewis point structure, or point electron structure) is a two-dimensional diagram used in chemistry to show the bonding between atoms of a molecule and the lone electron pairs that may be present in this molecule. It is ...
Lewis diagram for methane. Draw Lewis dot diagram for the following. Methane (CH4 ... Draw Lewis dot diagram for the following. Methane (CH4) Maharashtra State Board HSC Science (Computer Science) 11th. Textbook Solutions 6926. Important Solutions 17. Question Bank Solutions 4567. Concept Notes & Videos 336. Syllabus. Advertisement Remove all ads. Draw Lewis dot diagram for the following. ... Solved H (a) The Lewis structure for methane, CH4, is ... (a) Draw the best Lewis diagram for the molecule with the condensed formula CH3C (O)OCH. (b) There is a resonance structure for CH3C (O)OCHa for which all atoms other than H possess an octet of electrons. Draw this resonance structure. (e) As a thoughtexercise, use your answers for 3. (a) and 3. Methane Formula: Structure, Uses, Properties - Embibe Lewis Structure of Methane. Carbon belongs to group \(14\) of the Periodic Table and has \(4\) electrons in its valence shell. These electrons are represented by dots around the chemical symbol of carbon. As there are four valence electrons, it needs four more electrons to complete its octet configuration. Lewis Structure: Methane CH4 - YouTube Craig Beals shows how to draw the Lewis Structure for Methane.This is a clip from the complete video: Covalent Bonding 2.1 - Drawing Lewis Structures
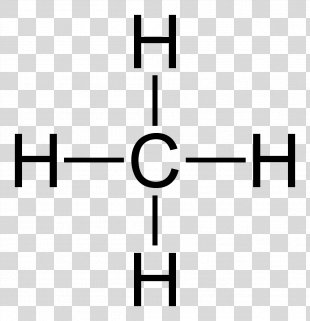
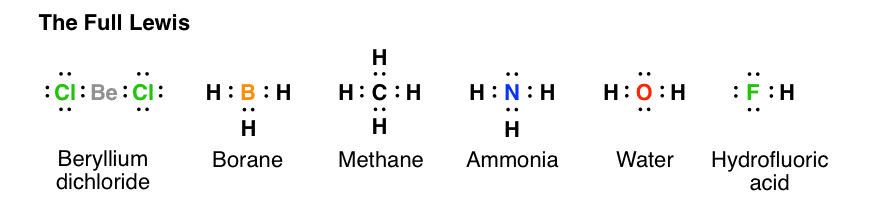
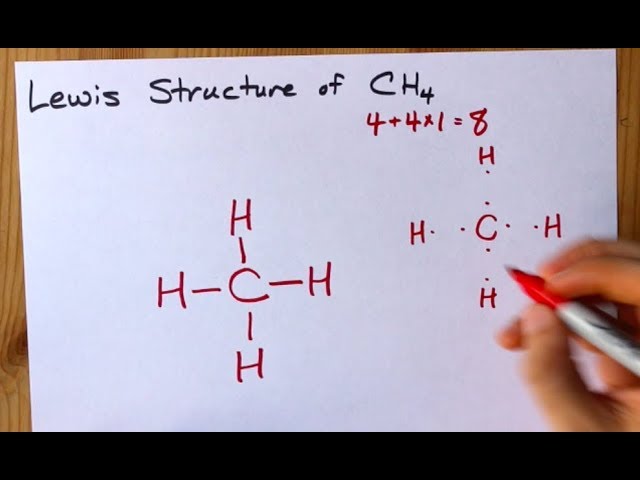
Lewis Structure for CH4 (Methane) - UMD Drawing the Lewis Structure for CH 4. For CH 4 you have a total of 8 total valence electrons. Drawing the Lewis structure for CH 4 (named methane) requires only single bonds. It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. What is the Lewis Structure for Methane? - Answers Hi, I am having trouble with chemistry an I need to know What the Lewis Structure for CH4 or Methane is an the number of groups on the central atom and the Number of bonding groups on the central ... Lewis Dot Structures 1. Methane 4 - HCC Learning Web Methane 4 Lewis Dot Structures 1. Methane - CH 4 Number of Valence Electrons: 4 from C and 1 each from 4 H = 8 Carbon is more electronegative than hydrogen, but hydrogen can never be the "central" atom, as it can only form 1 bond. Carbon always forms 4 bonds (2 electrons each). 2. Ammonia - NH 3 What is the Lewis structure of methane? | Study.com The Lewis dot structure for methane (CH 4) is: This is derived by following 5 general steps for writing Lewis dot structures. First, we need to... See full answer below.
Lewis Dot Diagram Ch4 Lewis Dot Structure for CH4 How to create a Lewis Dot Structure for CH4 # 2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1. How to draw the Lewis structure of methane, CH4 By José @ Periodic table with names diagramweb.net But seriously, you have an electron pair between the C and each of the H's in the ... CH4 Lewis Structure & Molecular Geometry - What's Insight Methane (CH4) is a colourless, odourless, and highly combustible gas that is utilized to generate energy and heat houses all over the world.CH4 Lewis structure comprises two different atoms: Carbon and hydrogen. It is a nonpolar molecule with a bond angle of 109.5° degrees. CH4 is utilized in chemical processes to create other essential gases such as hydrogen and carbon monoxide, as well as ... CH2Br2 Lewis Structure, Geometry, Hybridization, and ... The Lewis structure drawn in the above section is the most appropriate because it satisfies the octet rule and formal charges. The geometry and shape come out to be tetrahedral for dibromomethane (CH2Br2). Hybridization for the central atom in CH 2 Br 2 is sp 3. It is a polar compound. Happy Reading! Solved Draw the Lewis structure for the methane (CH4 ... Solved Draw the Lewis structure for the methane (CH4) | Chegg.com. Science. Chemistry. Chemistry questions and answers. Draw the Lewis structure for the methane (CH4) molecule. ء ☺ 0: | с 01 w х 5 ? Draw the Lewis structure for the selenium dioxide (SeO2) molecule. Be sure to include all resonance structures that satisfy the octet rule.
Methane - Wikipedia Methane's heat of combustion is 55.5 MJ/kg. Combustion of methane is a multiple step reaction summarized as follows: CH 4 + 2 O 2 → CO 2 + 2 H 2 O ( ΔH = −891 k J / mol, at standard conditions) Peters four-step chemistry is a systematically reduced four-step chemistry that explains the burning of methane. Methane radical reactions
Methane (CH4) lewis dot structure, molecular geometry ... The total valence electron available for drawing the Methane (CH4) lewis structure is 8. The steric number of the central atoms in methane is 4 which ensures that it has an Sp 3 hybridization. CH4 is a nonpolar molecule due to its symmetrical geometry that causes uniform charge distribution all over the atom leads to a zero net dipole moment and makes this molecule non-polar in nature.
Methane (CH4) Molecule Lewis Structure There are following specifications in the lewis structure of methane. Four hydrogen atoms have made bonds with center carbon atom and all those bonds are single bonds. No lone pairs on valence shells of carbon and hydrogen atoms. Oxidation number of carbon atom is -4. Steps of drawing lewis structure of CH 4 molecule
CH4 Lewis Structure, Molecular Geometry, and Hybridization The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.
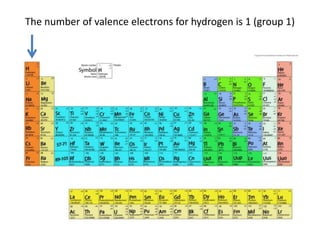
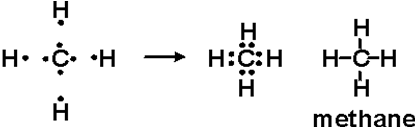
How to draw methane ch4 lewis structure - SlideShare Steps to write the Lewis structure of methane 1. Find the number of valence electrons for each of the atoms in the molecule. The number of valence electrons is usually the same as the group number where the element is located. 3. The number of valence electrons for hydrogen is 1 (group 1) 4. The number of valence electrons for carbon is 4 ...
CH4 Lewis Structure, Hybridization, Molecular Geometry ... Let us look at the Lewis Structure of CH4 and determine how the atoms are arranged in the molecule. Carbon in Methane takes the central position as it is less electronegative than the Hydrogen atoms. Arrange all the Hydrogen atoms around the Carbon atom. Now each Hydrogen just needs one more valence electron to attain a stable structure.
Electron Dot Diagram For Methane - schematron.org Mar 06, 2019 · Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) . Lewis dot dragram for methane: Methane, with molecular formula CH4, is shown. It is important to remember that Lewis valence dot diagrams are models that Methane is the main component of natural gas, and its chemical formula is CH4.
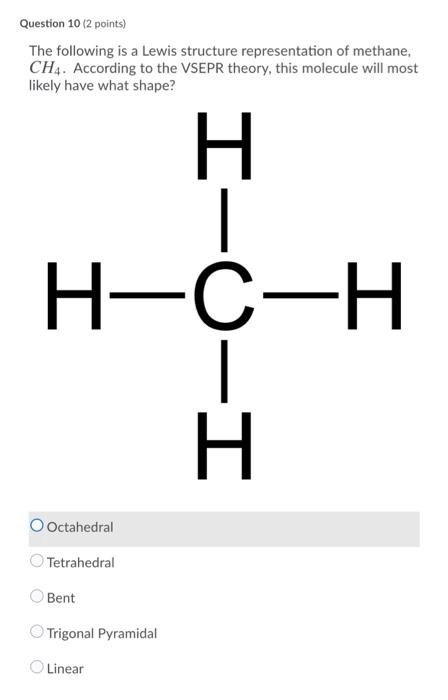
In a Lewis diagram for methane (CH4), which atom or atoms ... In the given molecule methane, CH₄: the valence configuration for C = 2s²2p² the valence configuration for H = 1s¹ Since two electrons are required to form a bond, the C atom in methane can form one bond with each of the 4 H atoms. Therefore, in the Lewis diagram, the C atom will be in the center surrounded by the 4 H atoms. Advertisement Survey
Methane: Molecular Geometry - Hybridization - Molecular ... Lewis structure: A Lewis structure or Lewis representation (also known as electron raster diagram, Lewis raster formula, Lewis point structure, or point electron structure) is a two-dimensional diagram used in chemistry to show the bonding between atoms of a molecule and the lone electron pairs that may be present in this molecule. It is ...
CH4 Lewis Structure - How to Draw the Dot Structure for ... How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use...
Methane | CH4 - PubChem Methane | CH4 | CID 297 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards ...
















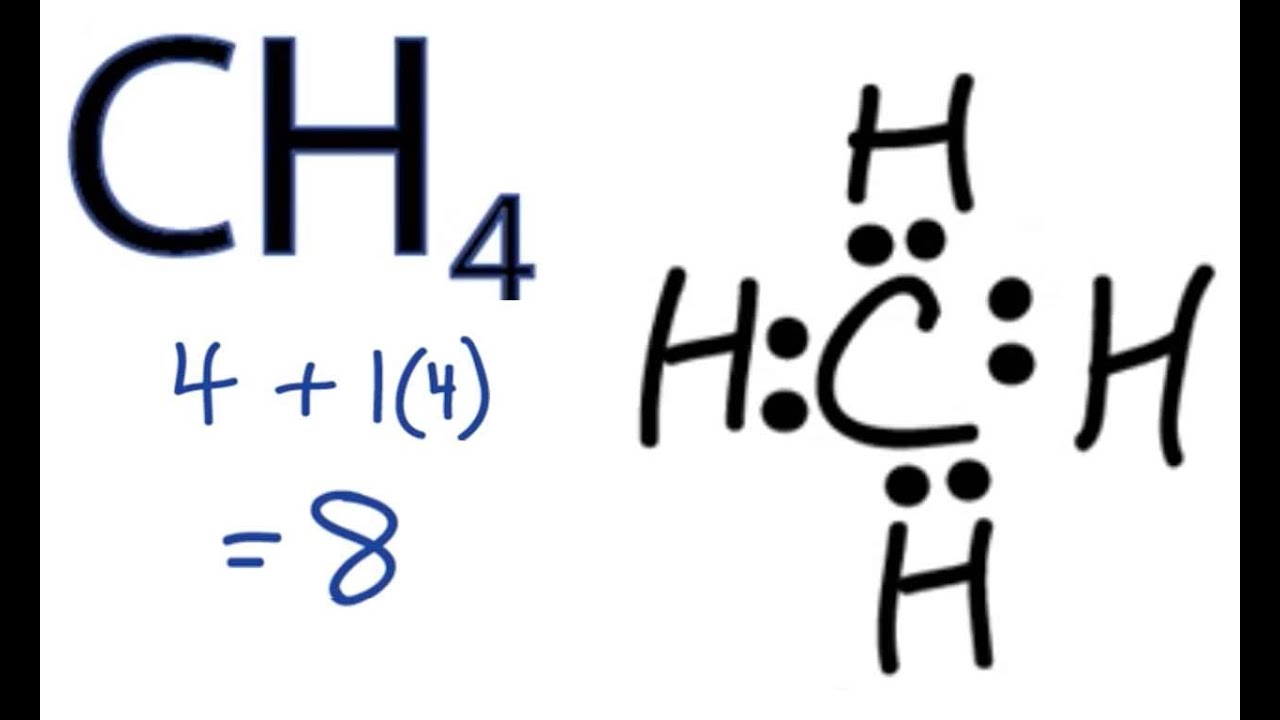


0 Response to "38 lewis diagram for methane"
Post a Comment