42 sulfur electron dot diagram
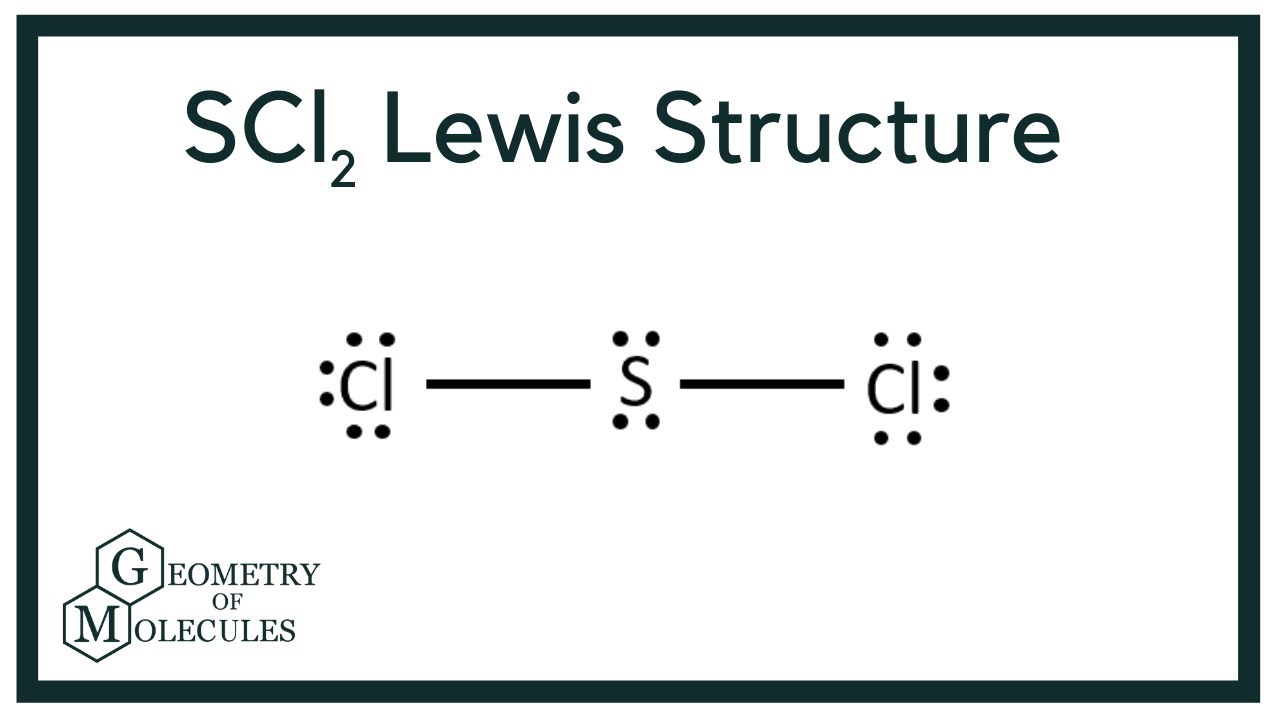
Lewis Dot Structure For Sulfur Dichloride - Novocom.top Lewis Dot Structure For Sulfur Dichloride, SCl2 Lewis Structure: How to Draw the Lewis Structure for, hexanedioyl dichloride 111 50 2, C6H8Cl2O2, density, Chemistry Chemical Bonding (9 of 35) Lewis Structures, VSEPR Theory: Valence Shell Electron Pair Repulsion Theory Sulfur Bohr Model - How to draw Bohr diagram for Sulfur (S ... Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Sulfur, we got to know, it has 6 valence electrons. So, just represent the 6 valence electrons around the Sulfur atom as a dot.
Cs2 Dot Structure Cs2 Dot Structure - The Lewis structure for CS2 requires you have double bonds between the Carbon C and Sulfur atoms in order to fill the octet of Carbon. After determining how many valence electrons there are in CS2 place them around the central atom to complete the octets. So2 Lewis Structure Chemistry Worksheets Chemistry Notes Electron Configuration

Sulfur electron dot diagram
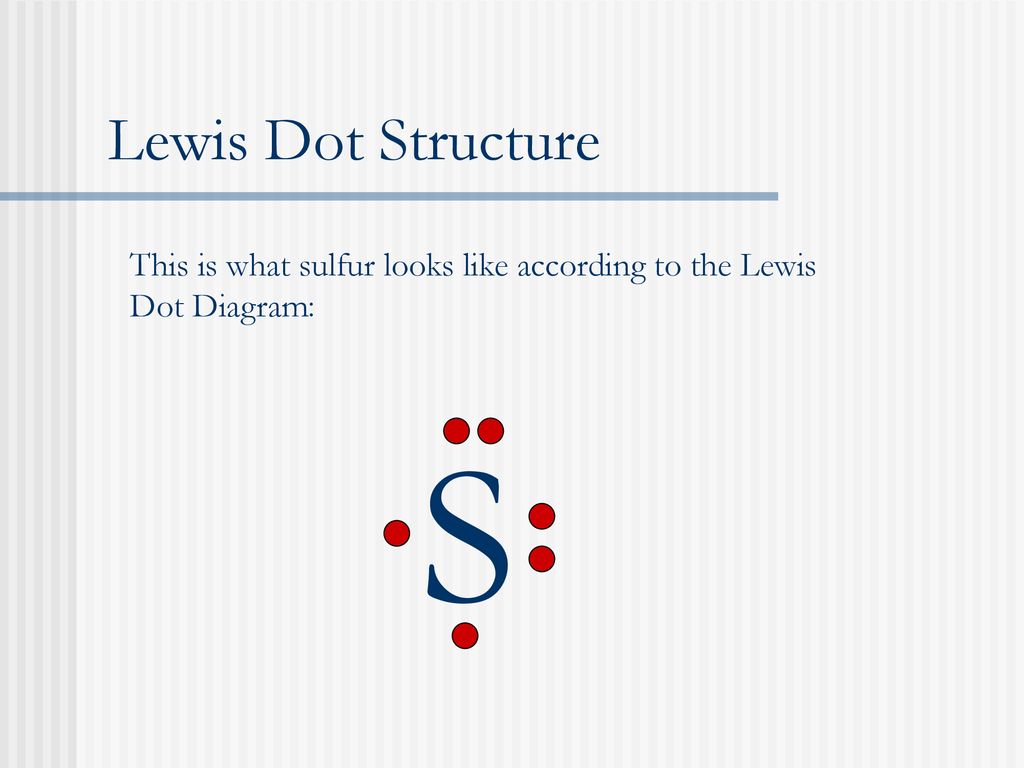
Electron dot diagram for sulfur? - Answers The electron dot diagram for a lone uncharged Sulfur particle is an S with 6 electrons arranged around it (2 orbitals with 2 electrons and 2 orbitals with 1). Electron Dot Diagrams and Lewis Structures - Quizlet electron dot diagram for Phosphorus. Lewis structure for PCl₃. Lewis structure for CH₄. Lewis structure for CH₃Br. Lewis structure for F₂O. Lewis structure for IBr. 6 dots around I, single bond, 6 dots around Br. Lewis structure for NH₂Cl. N in middle, 2 dots around N, single bonded to H on both left and right, single bonded down with ... 6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
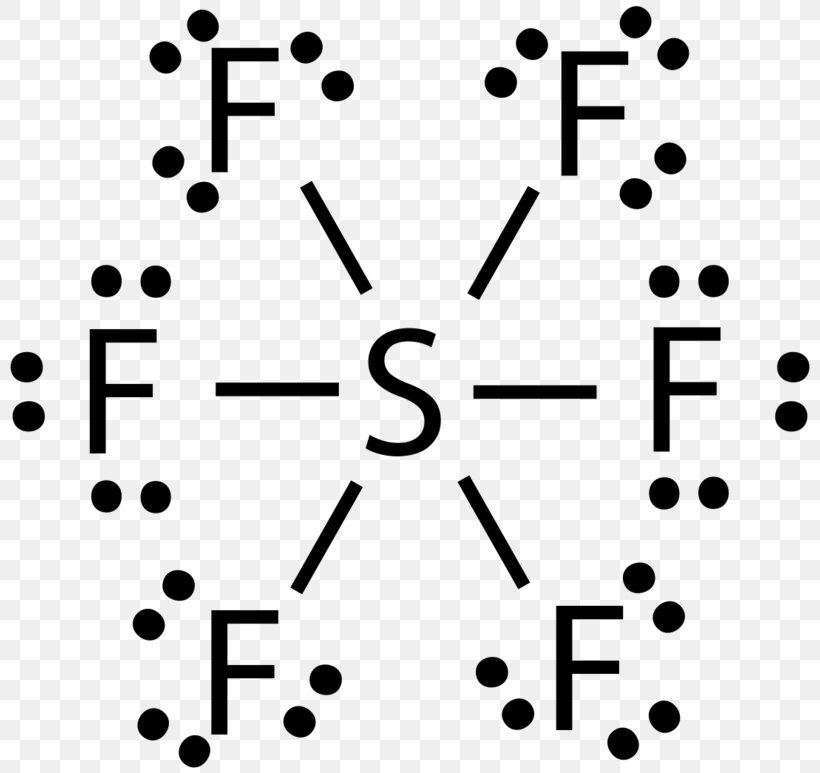
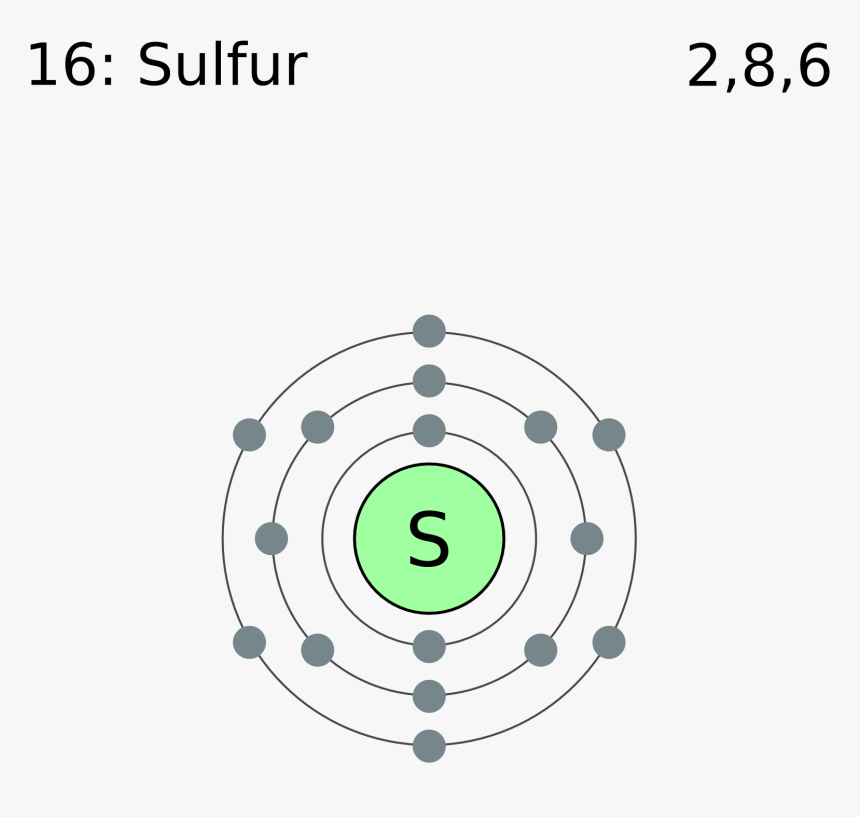
Sulfur electron dot diagram. Lewis dot diagram for sulfur What is the correct electron dot diagram for a neutral atom of SULFUR ? Answer : Surfum (s) electronic configuration, is? 252 286 352 3p 4 valence shell for Sulfur 352 384 The ne fone, electron dot diagram of a neutral sulfur is. :5: Sulfur And Chlorine Lewis Dot Diagram - Novocom.top Sulfur And Chlorine Lewis Dot Diagram, Show The Orbital Filling Diagram For Br Bromine Wiring, Lewis Dot Diagram For Potassium, How the ionic bond forms in Lithium Sulfide (Li2S) YouTube, Valence Shell Electron Pair Repulsion How to draw SBr2 Lewis Structure? - Science Education and ... SBr2 Lewis dot structure. To calculate the formal charge on the central sulfur atom of the SBr2 molecule by using the following formula: The formal charge on the sulfur atom of SBr2 molecule= (V. E(S)- L.E(S) - 1/2(B.E)) V.E (S) = Valence electron in a sulfur atom of SBr2 molecule (Get Answer) - Which is the electron dot structure of ... (A) Draw a Lewis Dot structure of selenium sulfur diiodide, SeSI_2, in which all atoms have an octet of electrons. (B) Calculate the formal charge of each atom in the structure in A. (C) Draw a resonance structure of SeSI_2 in which all atoms have a...
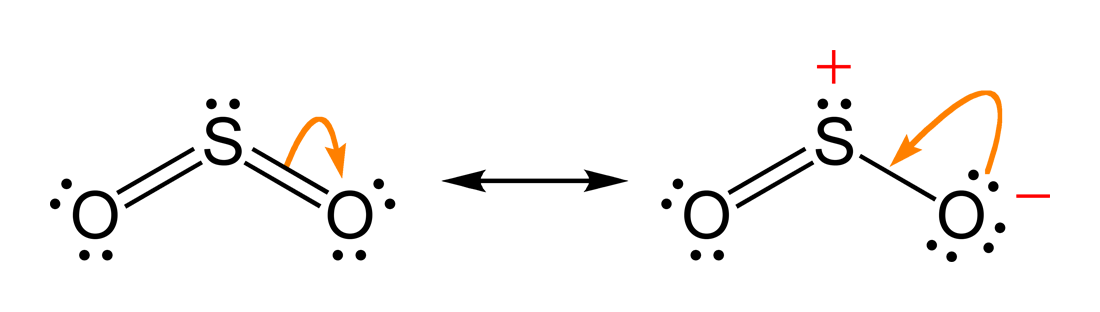
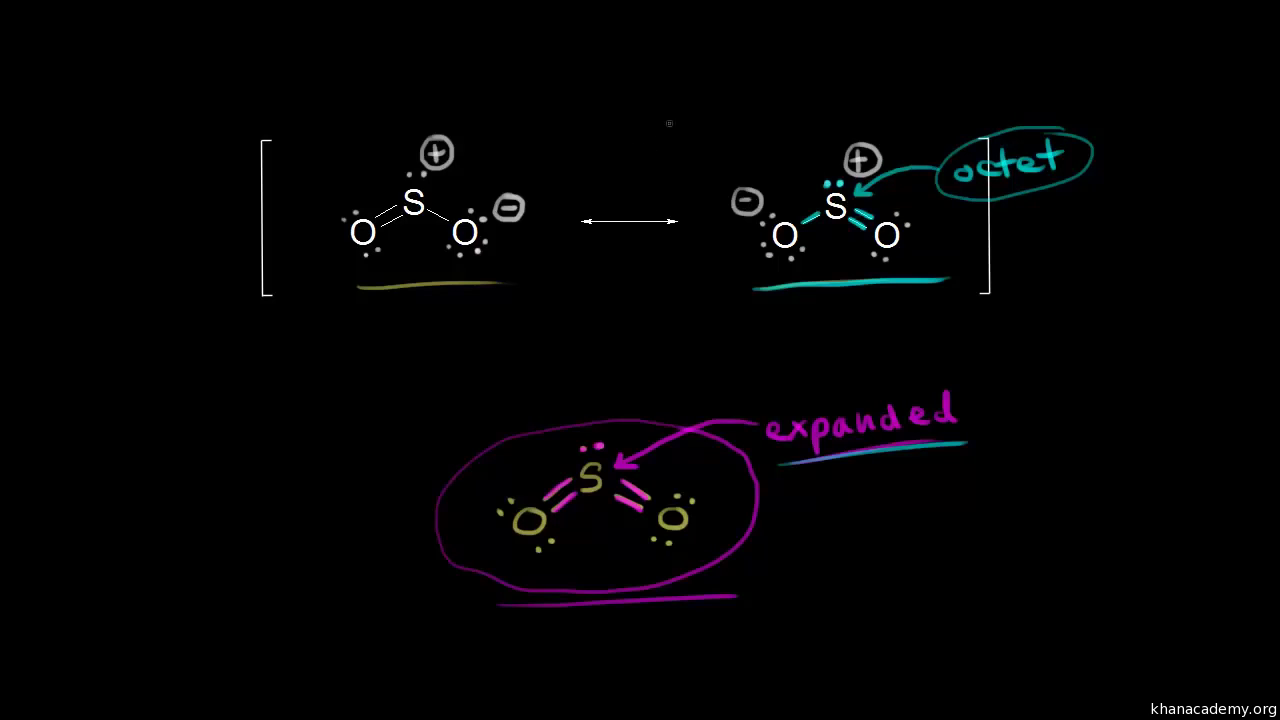
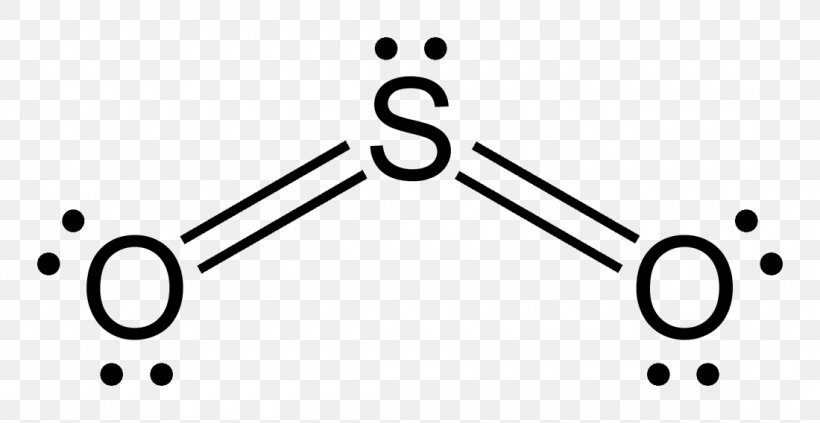
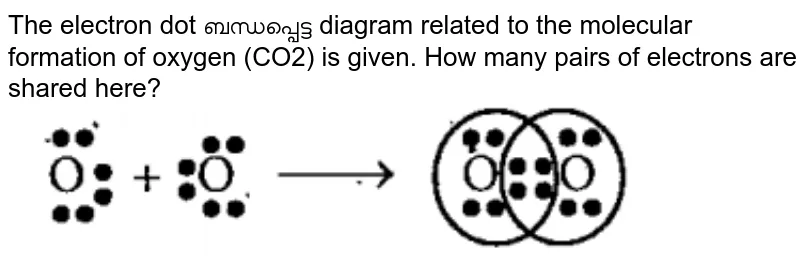
en.wikipedia.org › wiki › Octet_ruleOctet rule - Wikipedia Other rules exist for other elements, such as the duplet rule for hydrogen and helium, or the 18-electron rule for transition metals. The valence electrons can be counted using a Lewis electron dot diagram as shown at the right for carbon dioxide. Lewis Electron Dot Diagrams - Introductory Chemistry - 1st ... A. Lewis electron dot diagram. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... en.wikipedia.org › wiki › Oxidation_stateOxidation state - Wikipedia Electrochemical oxidation state [citation needed] represents a molecule or ion in the Latimer diagram or Frost diagram for its redox-active element. An example is the Latimer diagram for sulfur at pH 0 where the electrochemical oxidation state +2 for sulfur puts HS 2 O − 3 between S and H 2 SO 3: Sulfor dioxide: Lewis dot structure for SO2 (video) | Khan ... The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur.
Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ... topblogtenz.com › cyanide-cn-lewis-structureCN- lewis structure, molecular orbital diagram, and, bond order Also, using the Molecular orbital diagram of CN-we can also find its bond order which helps us to predict its bond length and stability as well. Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2. SCl2 Lewis Structure, Geometry, Hybridization, and ... SCl2 Lewis Structure. Lewis Structure or Lewis dot structure is one of the basic methods to determine the type of bonds between atoms. In this method, electrons in the valence shell are represented by dots, and two dots on different elements can be joined to form one bond. It is a 2-D representation of bonding. Lewis Dot Structure for Sulfur Atom (S) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for S (Sulfur). I show you where Sulfur is on the periodic table and how to determine how ...
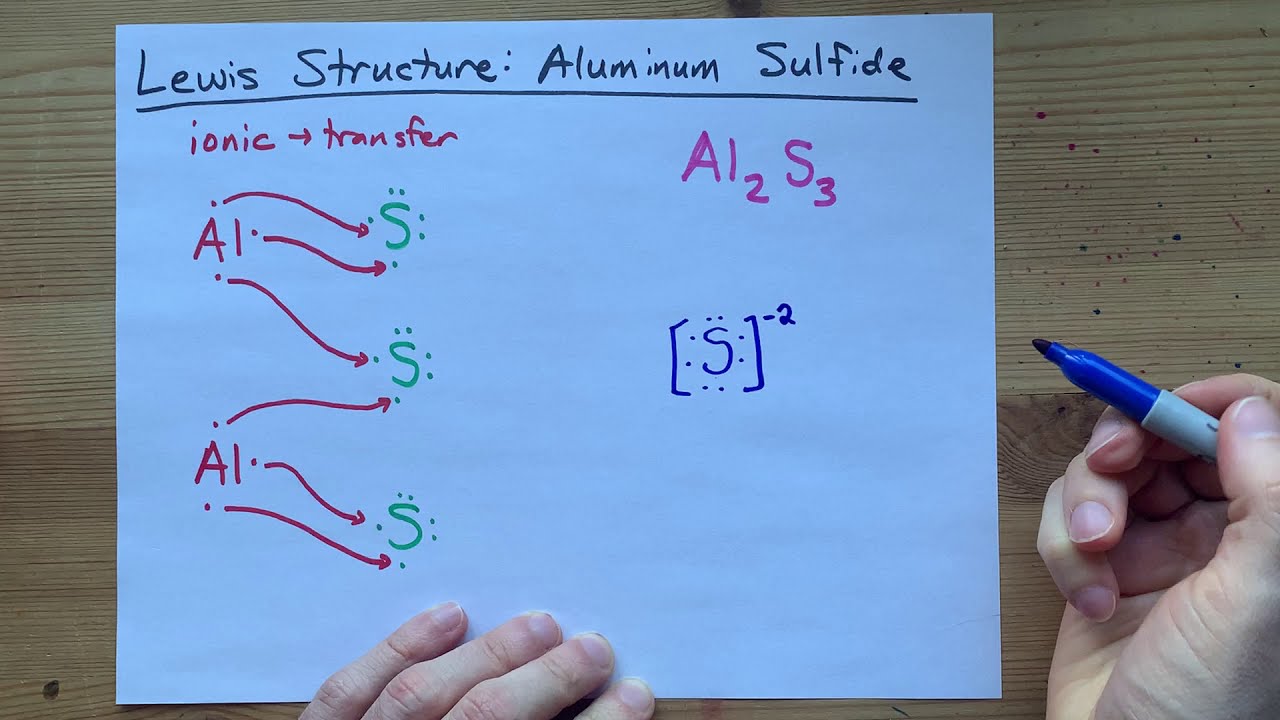
How to draw Lewis Dot Structure - Online Chemistry Tutor Lewis dot structure of Nitride ion. Now let us try Lewis dot structure of Sulfide ion ( S 2-).Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). [Ne]4s 2 4p 6. Valence electrons are 8 (2 in 3s and 6 in 3p) Lewis dot structure of sulfide ion
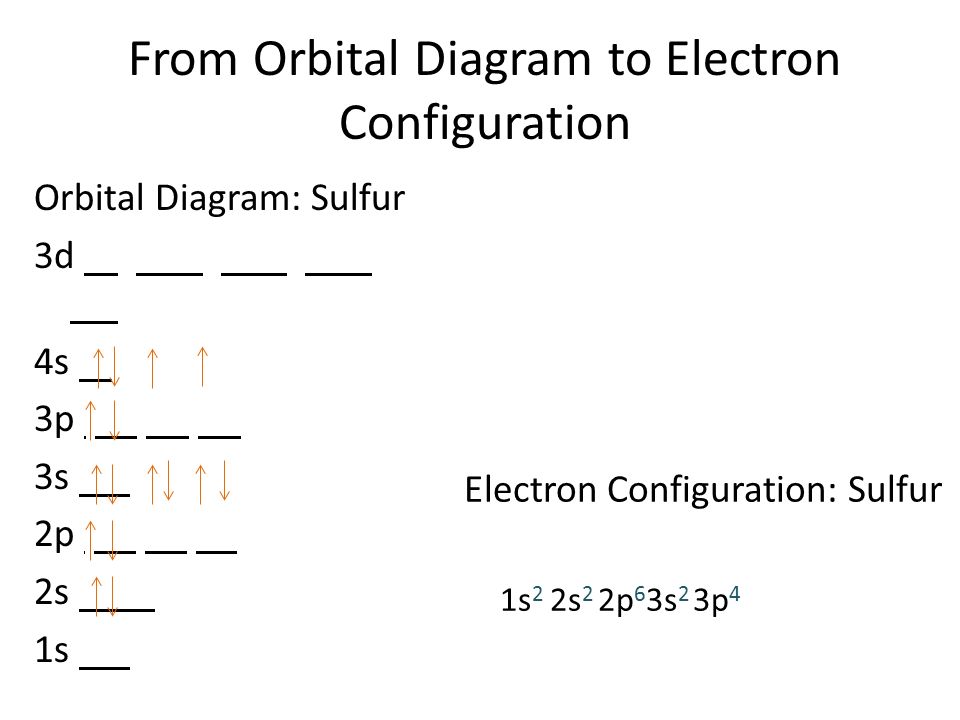
sarazscience.weebly.com › uploads › 1/8/0Electron Config & Orbital Filling Answer Key Electron Arrangements Name There are three ways to indicate the arrangement of electrons around an atom: 1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1.
(15) Lewis Diagrams (b) Sulfur dioxide. Sulfur dioxide, has a more complicated electron dot diagram. Presume that sulfur is in the middle because generally (but not always) the single atom in a molecular formula is the one that is usually in the center. Another generality is that the atom that needs the most electrons is in the center.
So2 Lewis Dot Diagram - schematron.org The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access . Drawing the Lewis Structure for SO 2.
How to draw CS2 Lewis Structure? - Science Education and ... In the CS2 Lewis structure diagram, the carbon atom can be the center atom of the molecule. As a result, central carbon in the CS2 Lewis structure, with all two sulfur atoms arranged in a linear geometry. Add valence electron around the sulfur atom, as given in the figure. Step-3: Lewis dot Structure for CS2 generated from step-1 and step-2
What is Lewis symbol sulfur? - Morethingsjapanese A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Electrons exist outside of an atom's nucleus and are found in principal energy levels that contain only up to a specific number of electrons.
opentextbc.ca › introductorychemistry › chapterAtomic Theory – Introductory Chemistry – 1st Canadian Edition Which is larger, a neutron or an electron? What are the charges for each of the three subatomic particles? Where is most of the mass of an atom located? Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus. Sketch a diagram of a helium atom, which has two protons and two neutrons in its nucleus. Define atomic ...
Electron Configuration for Sulfur (S) - UMD Therefore the sulfur electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 4. Video: Sulfur Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to ...
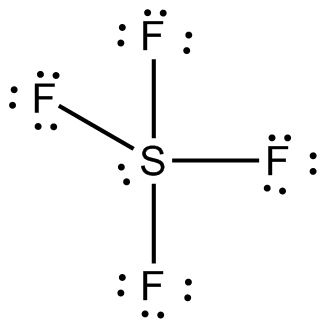
Lewis Dot Diagram For So4 2 - Wiring Diagrams Lewis Dot Diagram For So4 2. Simple procedure for drawing covalent Lewis structures - Lewis dot of the sulfate ion SO, best lewis structure for so, electron bonding. Viewing Notes: The Lewis structure for SO is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis.
Sulfur dibromide (SBr2) lewis dot structure, molecular ... SBr2 Lewis structure is made up of two atoms, sulfur, and bromine, the sulfur is in the central position and bromine atoms are in the surrounding position. The lewis structure of SBr2 contains 16 nonbonding electrons and 4 bonding electrons. The lewis structure of SBr2 is similar to the SCl2 and it is very easy to draw.
PDF Electron Dot (Lewis) Diagrams - Mr. Sault's Classroom 1. Draw an "electron dot" diagram showing the first 18 elements in the periodic table. 2. Explain how the electron dot diagram is similar for families in the periodic table. 3. Draw an electron dot diagram showing the formation of ions and ionic compounds. 4. Explain how hydrogen can be considered as behaving like a metal or a nonmetal.
PDF ELECTRONS Name electrons are the electrons in the ... sulfur . LEWIS DOT DIAGRAMS Name Lewis diagrams are a way to Indicate the number of valence electrons aroun an atom. A\5Ö are all examples of this type of diagram. Draw Lewis dot diagrams of the following . l. calcium 2. potassiürn rgon 4. aluminum 2-3 -B 5, bromine 6. carbon . 7. helium 8. oxygen 9. phosphorus . hy rogen . Created Date:
Dot Diagram For So2 - Wiring Diagrams Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. The Sulfur Dioxide which is also known as Sulphur Dioxide is the entity of a bond between Sulfur and Oxygen atoms.
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
Electron Dot Diagrams and Lewis Structures - Quizlet electron dot diagram for Phosphorus. Lewis structure for PCl₃. Lewis structure for CH₄. Lewis structure for CH₃Br. Lewis structure for F₂O. Lewis structure for IBr. 6 dots around I, single bond, 6 dots around Br. Lewis structure for NH₂Cl. N in middle, 2 dots around N, single bonded to H on both left and right, single bonded down with ...
Electron dot diagram for sulfur? - Answers The electron dot diagram for a lone uncharged Sulfur particle is an S with 6 electrons arranged around it (2 orbitals with 2 electrons and 2 orbitals with 1).





![Expert Verified] Write electron dot structure of calcium and ...](https://hi-static.z-dn.net/files/d24/866245ecdbd6e4ecd924be4705ee77c2.png)


/Lewis-dot-58f78f405f9b581d5938e617.jpg)
















0 Response to "42 sulfur electron dot diagram"
Post a Comment