42 reaction coordinate diagram endothermic
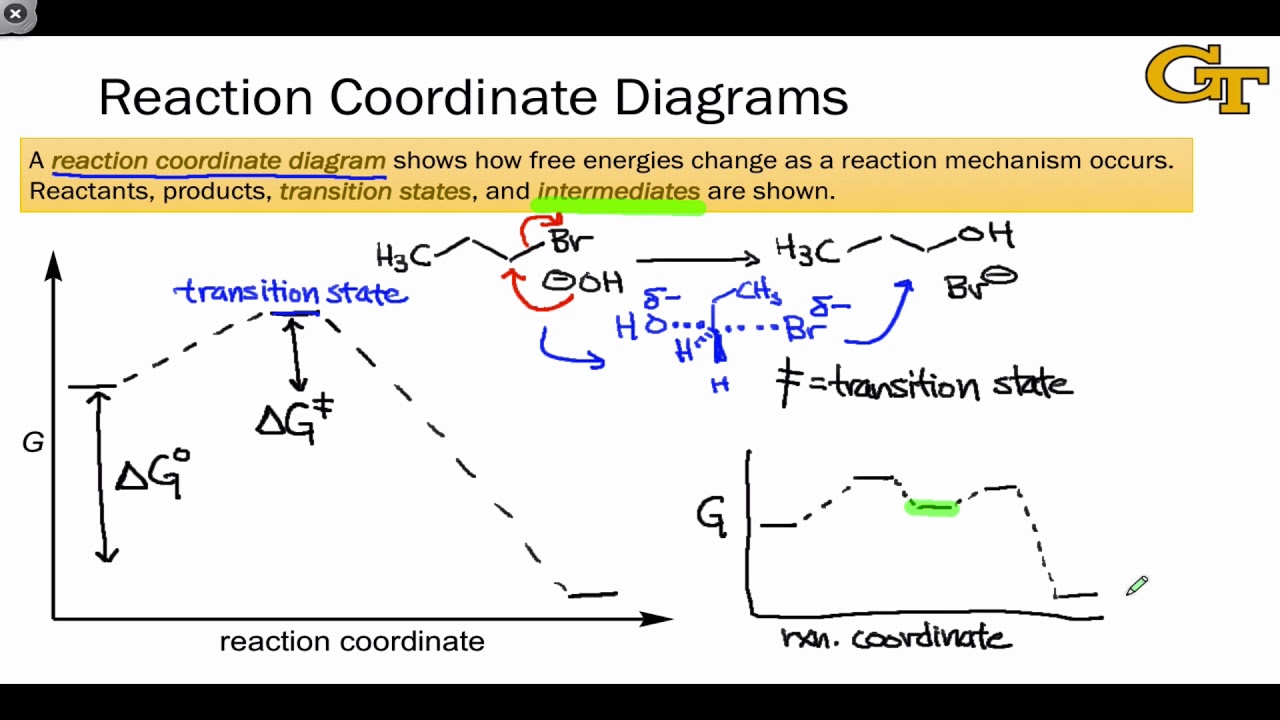
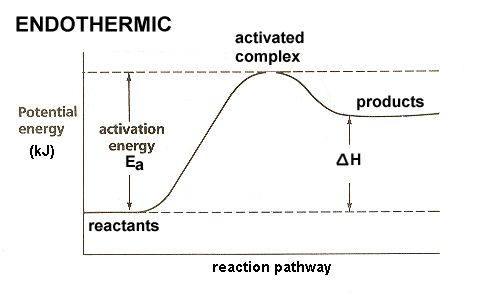
PDF Chapter 5. Reactions of Alkenes and Alkynes Learning ... Chapter 5. Reactions of Alkenes and Alkynes Learning objectives: 1. Identify the followings from a reaction coordinate diagram when applicable: endothermic or exothermic reactions, activation energy, heat of reaction, locations of transition states, locations of intermediates, and rate-limiting step. 2. Arrhenius Theory and Reaction Coordinates Typically, we envision reactions proceeding left to right along the reaction coordinate, so often, the activation energy is only noted for the forward reaction. The activation energy on the diagram below shows the barrier to be 102.6 kJ mol -1 .
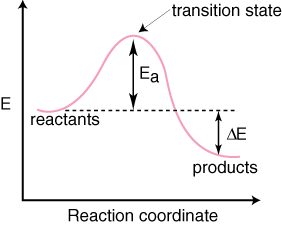
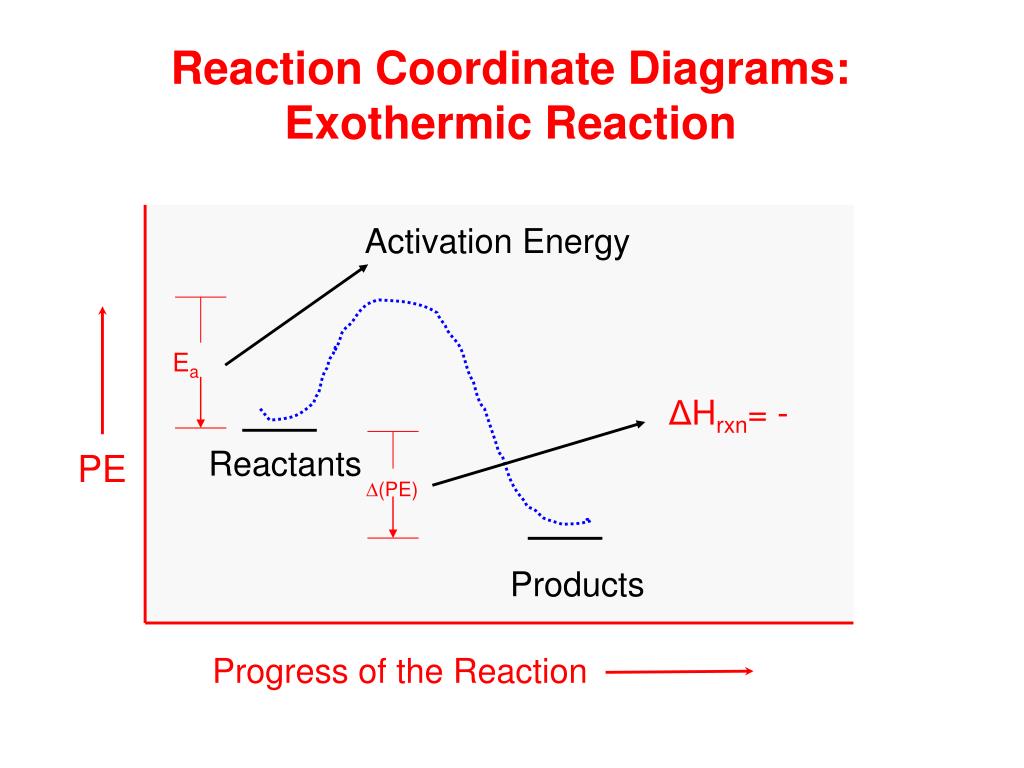
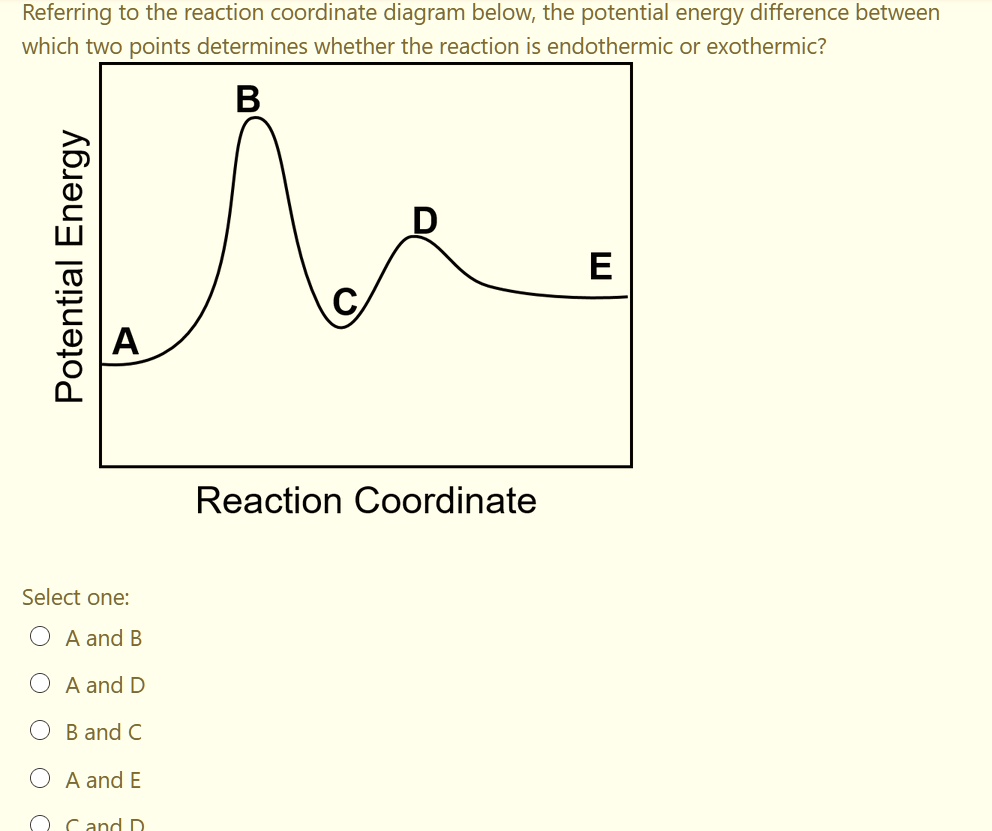
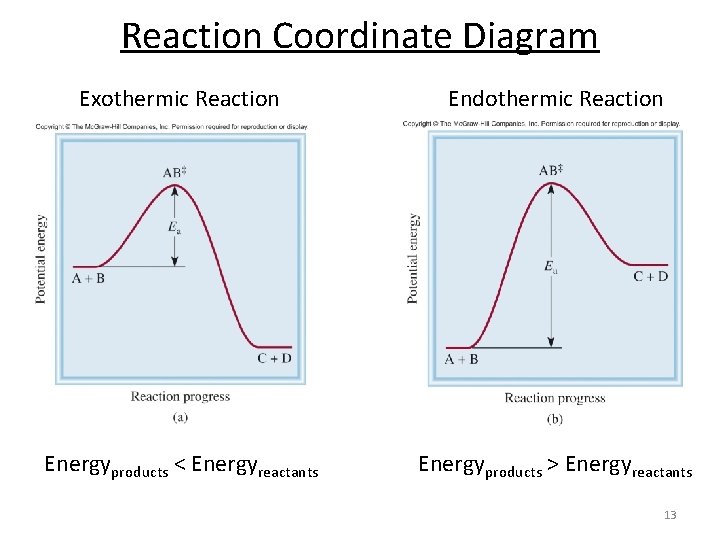
Reaction Coordinate Diagrams - University of Illinois ... The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction(heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram.
Reaction coordinate diagram endothermic
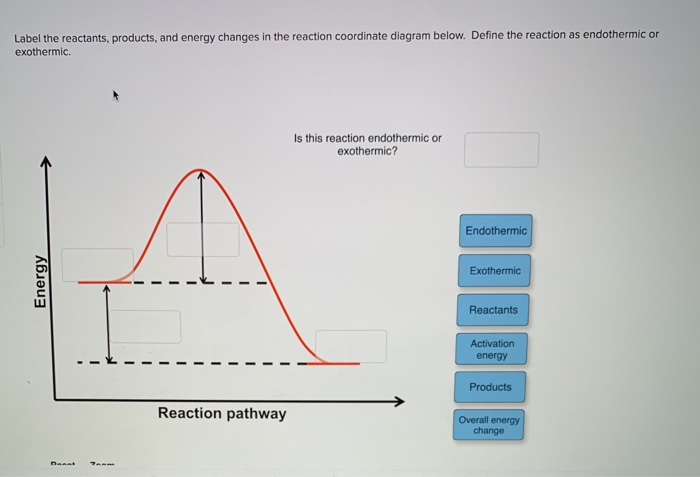
PDF Additional Problems for practice: 1. Draw a reaction ... 1. Draw a reaction energy diagram (graph of potential energy versus reaction coordinate) for a three-step endothermic reaction with the (a) first step rate-determining (b) the second step rate-determining (c) the third step rate determining Do the same (a-c) for a three step exothermic reaction. For each graph, PDF Exothermic Endothermic "downhill" "uphill" 7. Be able to recognize and read potential energy diagrams. Reaction Coordinate Reaction Coordinate Exothermic Endothermic "downhill" "uphill" 8. ∆H is (+) for endothermic reactions and is (-) for exothermic reactions. 9. The rates of the forward and reverse reactions are equal at equilibrium. 10. Reaction Coordinate Diagrams - College Chemistry Explanation: The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants.
Reaction coordinate diagram endothermic. Analyzing Energy With a Reaction Coordinate Diagram ... And a reaction coordinate diagram where the energy level of B ends up lower than A is exothermic (delta E is negative): Activation Energy The activation energy is important in a reaction. Even if... Endothermic Reaction Coordinate Diagram - schematron.org The fully filled in reaction coordinate diagram is displayed below. This reaction is also exothermic because the energy of the products is lower than that of the. In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other.Start studying CHEMISTRY 2. Drawing reaction coordinate diagrams for exothermic and ... PDF imarkic.weebly.com tables to determine if this reaction is endothermic or exothermic. Construct a reaction coordinate diagram that shows the endothermic or exothermic nature of the reaction and illustrates why this reaction is under kinetic control. Each diagram below (I, Il, Ill, IV) describes a possible reaction: 2 AB(g) A2(g) + B2(g)
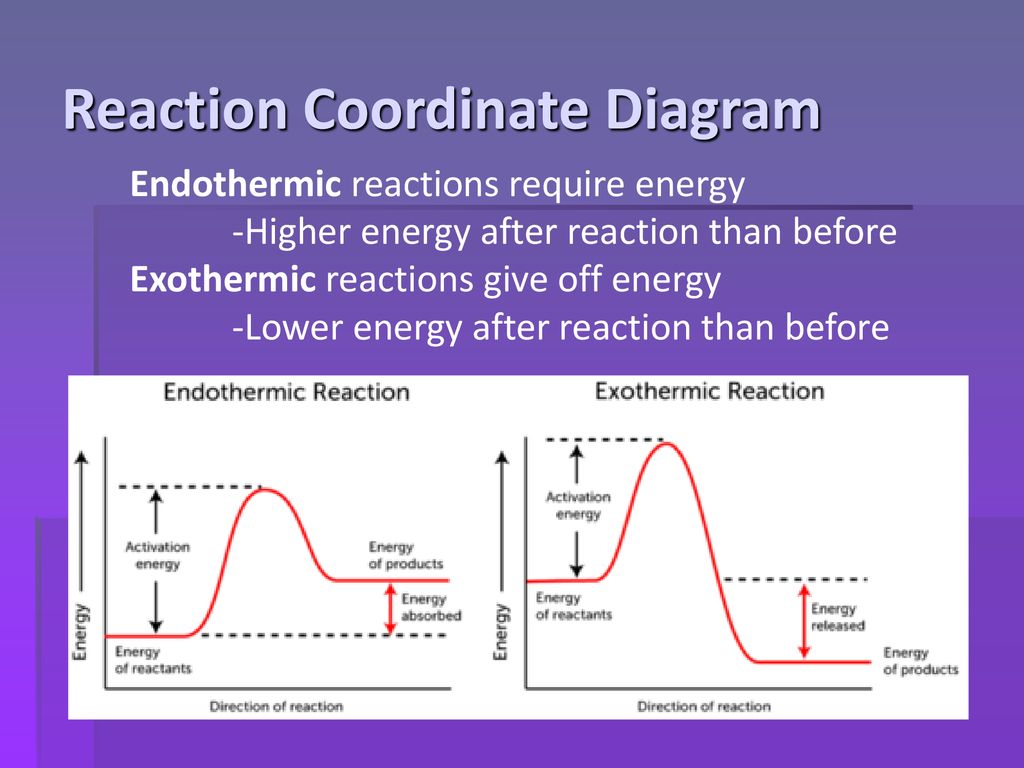
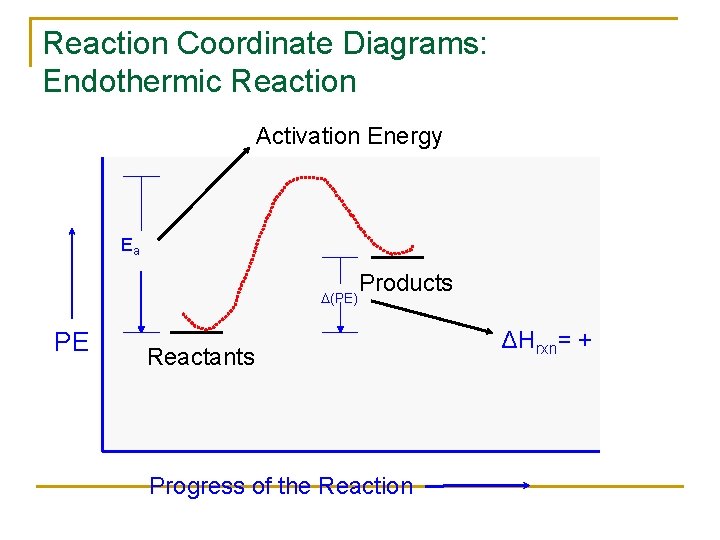
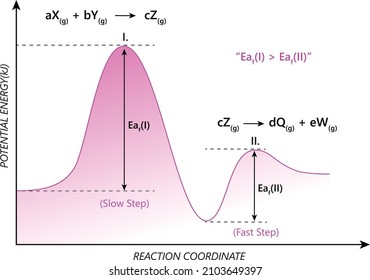
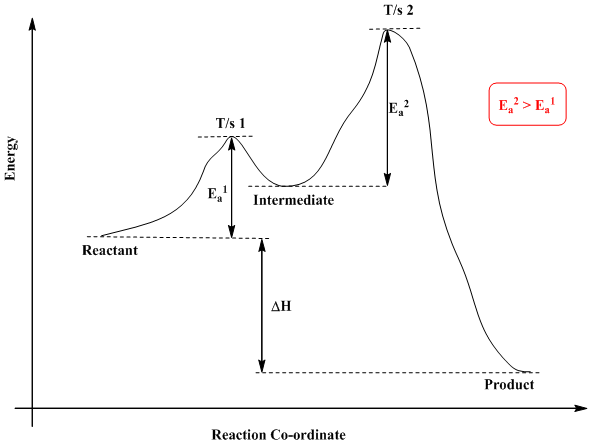
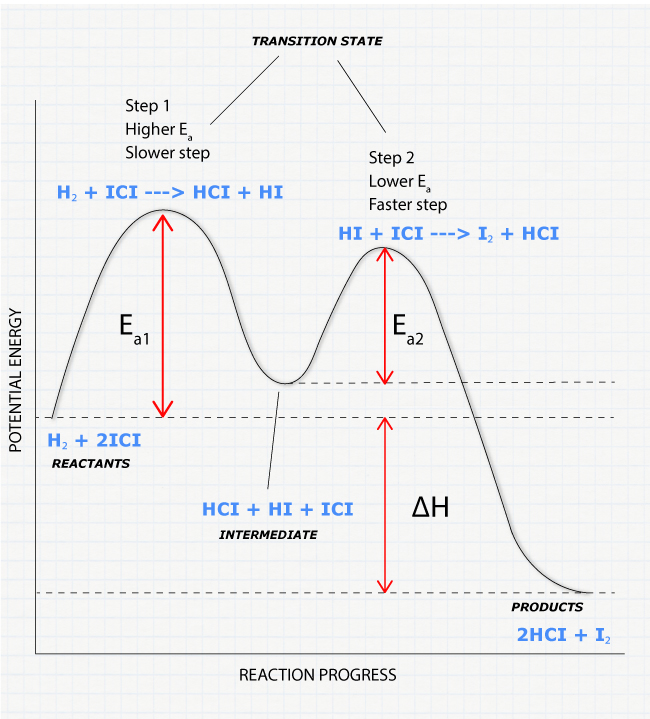
6. Reaction Coordinate Diagram - VIZISCIENCE® INTERACTIVE LABS 6. Reaction Coordinate Diagram. Given the following reaction, sketch a reaction coordinate graph. The reaction involves two steps, step 1 is the slowest step and step 2 is the fastest step. Both steps are exothermic. Indicate on the diagram the overall enthalpy change of the reaction, the reaction for the transition states and intermediate states. Endothermic Reaction Coordinate Diagram - Wiring Diagrams A reaction is endothermic when the energy of the products is greater than the energy of the reactants. The is for an exothermic reaction. Below is a reaction coordinate diagram for an endothermic reaction. A reaction coordinate diagram shows the energy changes that take place in each of the steps of the mechanism. 5.3. Reaction coordinate diagrams | Organic Chemistry 1 ... In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction from starting compounds to final products. SOLVED:Draw reaction coordinate diagram for slow ... Draw reaction coordinate diagram for slow, concerted endothermic reaction: Label axes_ reactant, product, transition state, activation energy and AG. Draw reaction coordinate diagram for slow stepwise reaction that favors reactants.
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In endothermic reactions, there is less energy in the reactants than ... Reaction Coordinate Diagram Endothermic - Wiring Diagrams The " reaction coordinate " plotted along the abscissa represents the diagrams can describe both exothermic and endothermic reactions.A reaction will be exothermic if the energy of the products is less than the energy of the reactants. A reaction is endothermic when the energy of the products is greater than the energy of the reactants. PDF Energy/Reaction Coordinate Diagrams 1! Energy/Reaction Coordinate! Diagrams! Thermodynamics, Kinetics ! Dr. Ron Rusay" A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction What are Endothermic Reactions? (with Examples & Video) The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant.
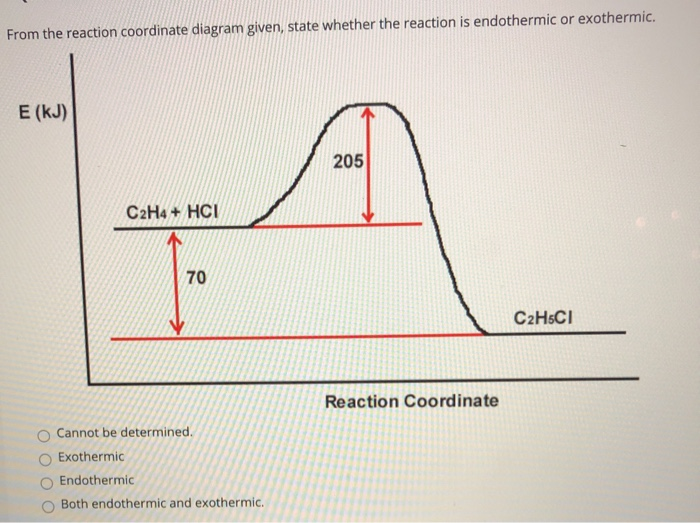
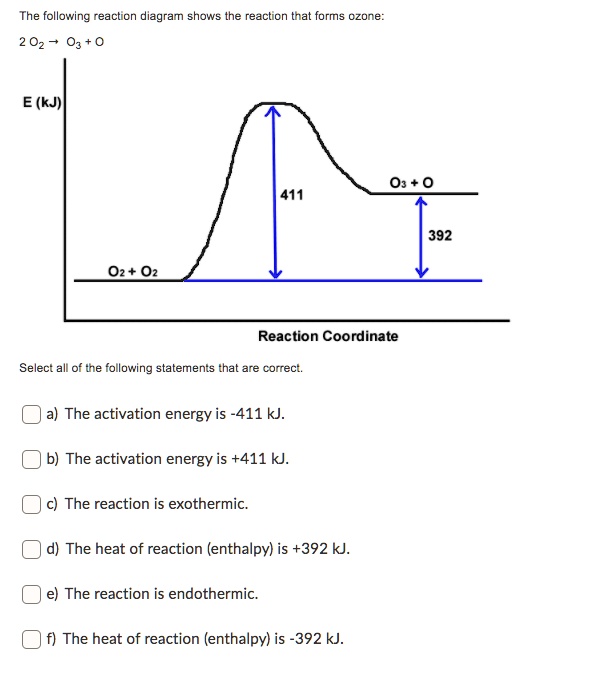
Interpreting a reaction energy diagram Consid | Chegg.com Here is an energy diagram for the reaction: 100 CD 4 + 8 energy (kal/mol) 200 100 0 reaction coordinate Use the energy diagram to answer these questions. kJ/mol What is the heat of reaction? Exothermic Endothermic Neither Is the reaction exothermic or endothermic? Yes, it's [] kJ/mol No.
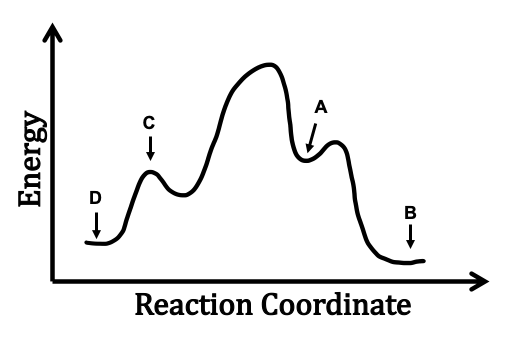
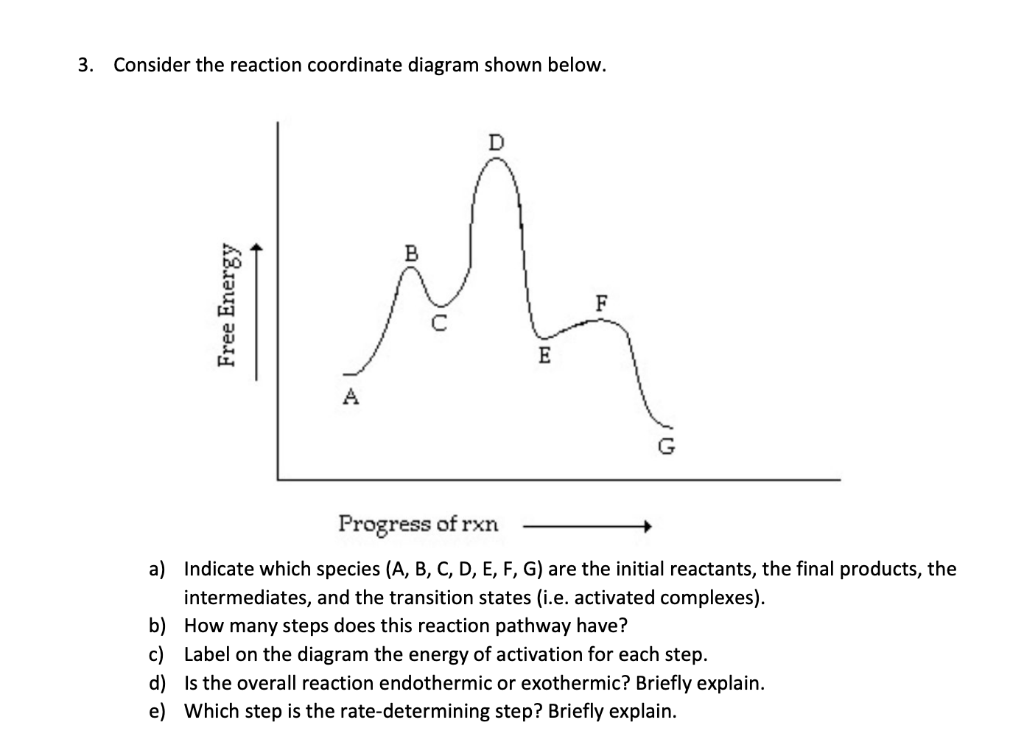
Answered: 1. a) Write the reaction profile… | bartleby a) Write the reaction profile (reaction coordinate diagram) for an endothermic reaction that occurs via a 3 step mechanism with the second step being the rate determining step? b) Label reactants and products, each activation energy and the enthalpy change for the reaction. c) Label the location of intermediates.
Reaction Coordinate Diagram practice problems Quiz - Quizizz What does arrow #1 represent in this reaction coordinate diagram represent? What does arrow #3 represent in this reaction coordinate diagram represent? What is the change in enthalpy (ΔH rxn) of the reaction in the picture? What is the potential energy of the products (PE products) in the graph in the picture?
Answered: Consider the potential energy vs… | bartleby Consider the potential energy vs reaction coordinate diagram in problem 70 in your text on pg 616. Which of the answers below best describes the change in enthalpy for this reaction? Group of answer choices A) thermoneutral B) endothermic C) exothermic D) endogonic. Consider the potential energy vs reaction coordinate diagram in problem 70 in ...
What is a reaction coordinate diagram? - FindAnyAnswer.com Reaction Coordinate Diagrams.Let's consider a general reaction where a reactant or set of reactants, A, is transformed into a product or set of products, B. The diagram below is called a reaction coordinate diagram.It shows how the energy of the system changes during a chemical reaction.
PDF Thermodynamics vs Kinetics - Columbia University A general Reaction Coordinate Diagram relating the energy of a system to its geometry along one possible reaction pathway is given in the figure below. In the figure below, the Activation Energy, Ea is that critical minimum energy in a chemical reaction required by reactants to be converted into products. the quantities, Ea;
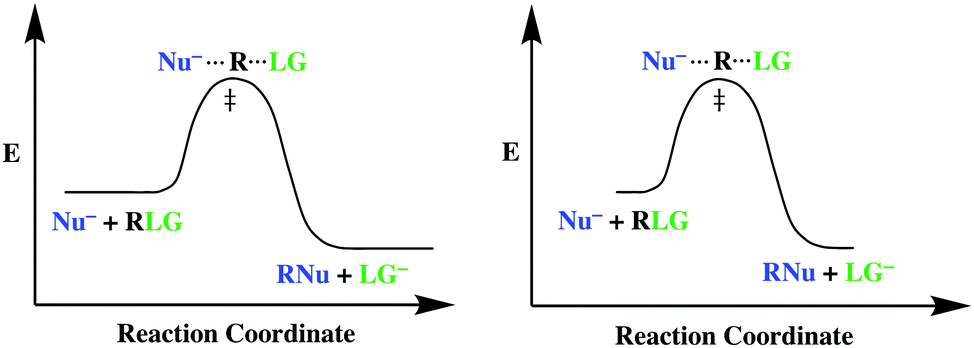
PDF Reactions of Alkenes - University of Texas at Austin • Energy diagram: A graph showing the changes in energy that occur during a chemical reaction. • Reaction coordinate: A measure in the change in positions of atoms during a reaction. Reaction coordinate Energy Energy Diagrams 6 • Transition state ‡: - An unstable species of maximum energy formed during the course of a reaction.
Solved Potential Energy Reaction Coordinate The forward ... Chemistry. Chemistry questions and answers. Potential Energy Reaction Coordinate The forward reaction that this diagram describes is Select one: exothermic O a. O b. endothermic O c. neither exothermic nor endothermic O d. there is not enough information to tell, because the axes are not labeled with proper units Check.
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Solved 3. Based on the following energy diagram, is the ... Chemistry. Chemistry questions and answers. 3. Based on the following energy diagram, is the reaction exothermic or endothermic? How many steps are in this reaction? How many transition states and intermediates? free energy, kJ/mol reaction coordinate 4. Based on the following energy diagram, is the reaction exothermic or endothermic?
Reaction Coordinate Diagram Endothermic Vs Exothermic A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change there are many ENDOTHERMIC reactions such as photosynthesis that occur.! An Exothermic (Exergonic) Reaction! 2 C 8 H 18 + 25 O 2 → 16 CO 2 + 18 H 2 O! A reaction with ∆H°0 is endothermic.
Reaction Coordinate Diagrams - College Chemistry Explanation: The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants.
PDF Exothermic Endothermic "downhill" "uphill" 7. Be able to recognize and read potential energy diagrams. Reaction Coordinate Reaction Coordinate Exothermic Endothermic "downhill" "uphill" 8. ∆H is (+) for endothermic reactions and is (-) for exothermic reactions. 9. The rates of the forward and reverse reactions are equal at equilibrium. 10.
PDF Additional Problems for practice: 1. Draw a reaction ... 1. Draw a reaction energy diagram (graph of potential energy versus reaction coordinate) for a three-step endothermic reaction with the (a) first step rate-determining (b) the second step rate-determining (c) the third step rate determining Do the same (a-c) for a three step exothermic reaction. For each graph,



















0 Response to "42 reaction coordinate diagram endothermic"
Post a Comment