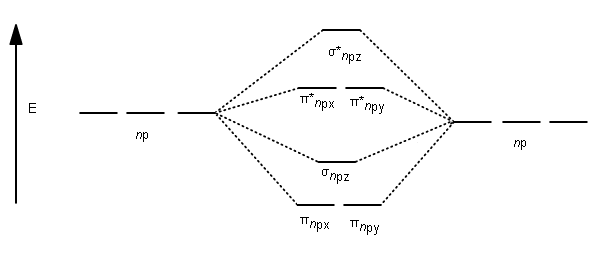
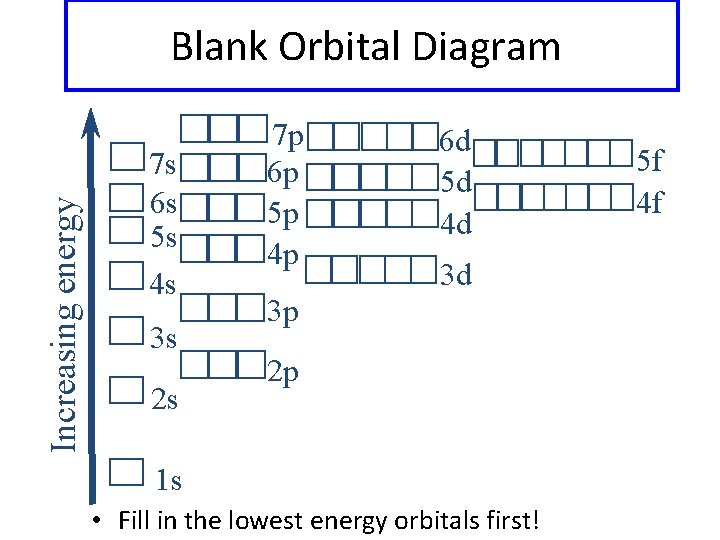
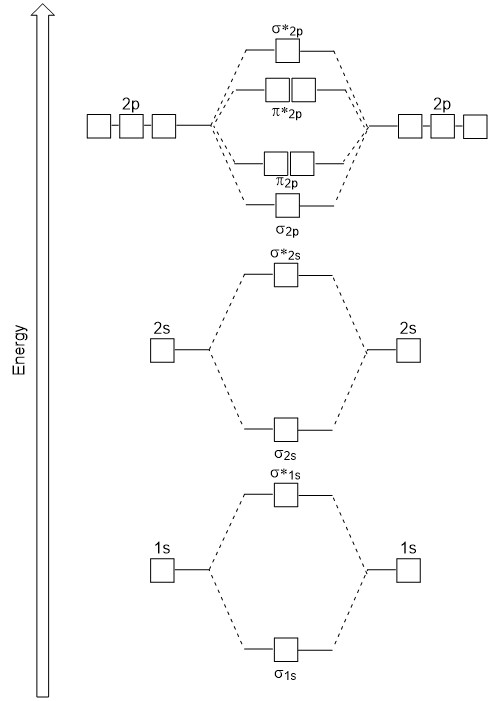
42 blank molecular orbital diagram
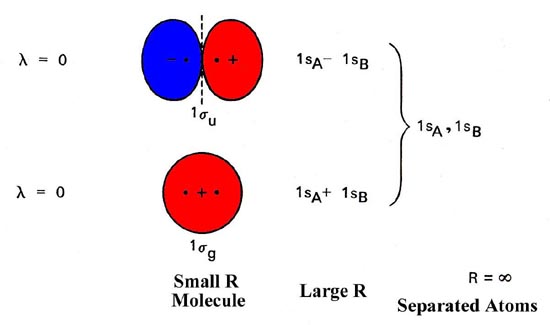
An introduction to molecular orbital theory The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive... Molecular Orbital Theory Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the geometry of the molecule to which they are applied. This diagram suggests that the energy of an H2 molecule is lower than that of a pair of isolated atoms.
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... - Molecular orbital are formed by addition and subtraction of AO's. Æ Linear Combination of Atomic Orbitals (LCAO). - like hybrid AO's but the • Energy level diagram represents this interaction. - Two s orbitals interaction to create a low energy bonding and high energy anti-bonding molecular orbital.
Blank molecular orbital diagram
Structural Biochemistry/How to Construct Molecular Orbital Diagram... 1. First, determine the point group of the molecule. 2. Draw out the orbitals of the central atoms. 3. Determine the irreducible representation of the orbitals of the central atoms. 4. Draw out the possible bonding interaction of the orbitals from the peripheral atoms. Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the... Orbital Diagrams and Electron Configuration - Basic Introduction... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram...
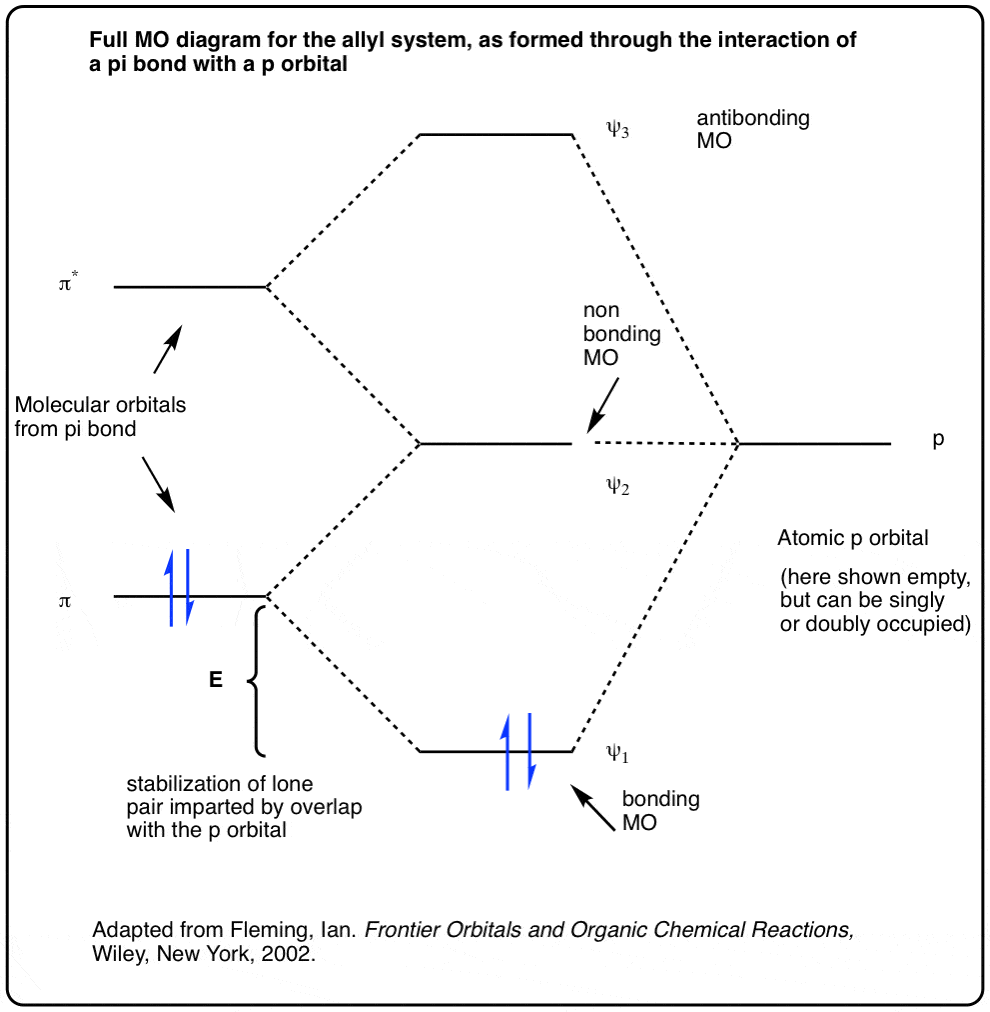
Blank molecular orbital diagram. MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available Molecular Orbital Theory: Definition, Postulates, Formation, and Types Molecular Orbital Theory: It is used to define the bonding in molecules which cannot be explained with the Molecular Orbital Theory: To simplify things, we will consider the interaction of the orbitals containing or in terms of energy level diagram, it is represented as below. Here, Nb=4,Na=4. So that. The Pi Molecular Orbitals of Butadiene And How To Draw Them The Lowest-Energy Molecular Orbital (π1) Of The Butadiene Pi System Has Zero Nodes The Full Molecular Orbital Diagram For The Butadienyl System (n=4) A molecular orbital diagram without electrons is like an apartment building without people. Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
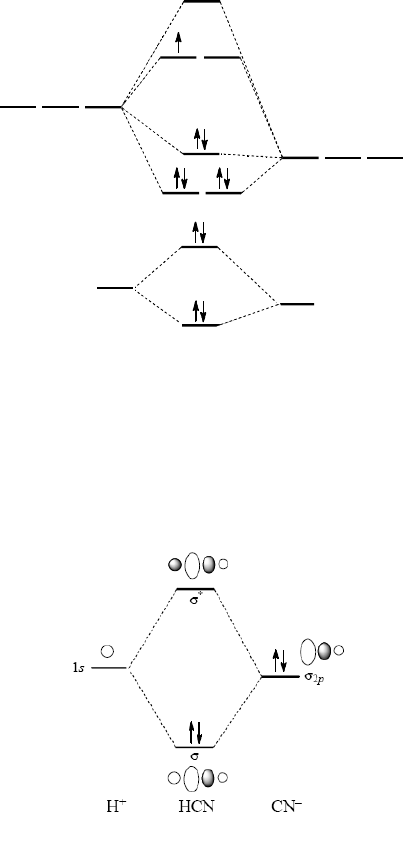
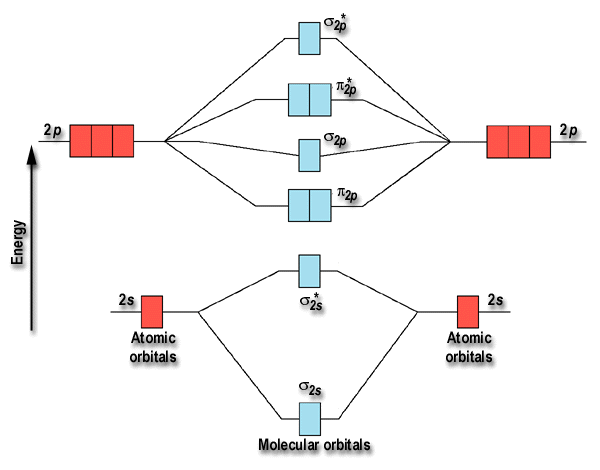
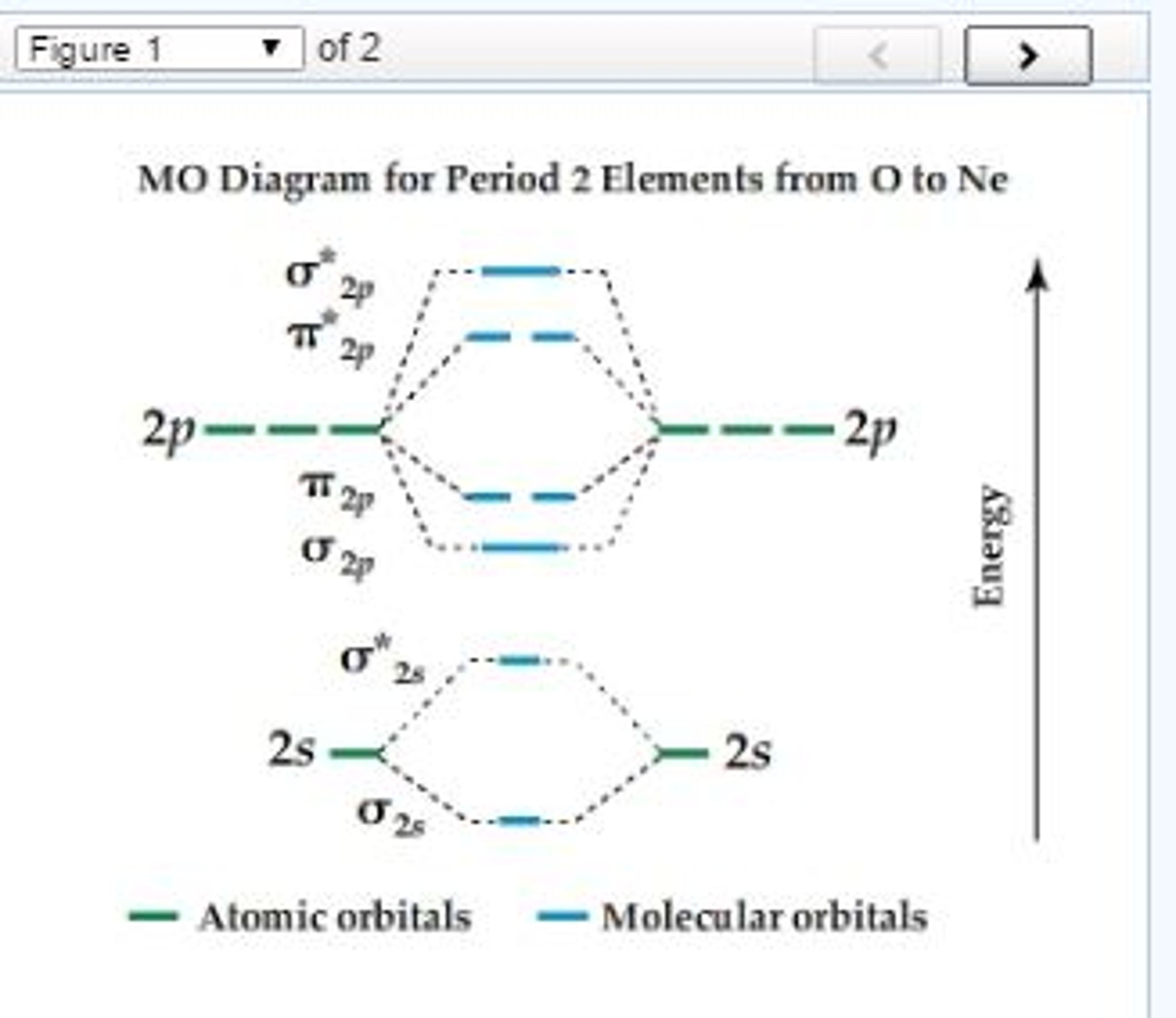
PDF Figure 9.32: The molecular orbital energy-level diagram for • The following slide illustrates the relative energies of the molecular orbitals compared to the original atomic orbitals. • Because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. Figure 9.26: (a) The molecular orbital energy-level diagram for the... What is the molecular orbital diagram for HCL? - Quora Molecular orbital energy level diagram of CO molecule can be given as. CO molecule has 10 valence electrons,four from carbon atom (2s²2p²) and six from oxygen atom (2s²2p⁴).According to molecular orbital diagram, molecular orbital configuration is given as. PDF Molecular Orbital Theory Molecular Orbital Theory. In order to simplify things, we'll consider the interaction of the orbitals containing valence electrons to create molecular orbitals. The wave functions of hydrogen atom A and hydrogen atom B can interact either constructively or destructively. PDF Molecular | 90" (porbitals). This dilemma has been resolved by orbital IN THE APPLICATION of molecular orbital theory to calculations of chemical binding energies, we shall use s e v e r a l basic principles, some of which w e r e mentioned in Consider azulene, which has the following "molecular diagram": Clearly an electrophilic reagent would be expected to react a t.
Molecular Orbitals: Molecular Orbital Theory | SparkNotes Molecular orbital theory posits the notion that electrons in molecules likewise exist in different orbitals that give the probability of finding the electron at Notice that the orbitals of the separated atoms are written on either side of the diagram as horizontal lines at heights denoting their relative energies. How to Do Orbital Diagrams | Sciencing Orbital diagrams give you all of the information you need about the electron configuration and occupied spin states for chemistry or physics, and are easy Dot diagrams are very different to orbital diagrams, but they're still very easy to understand. They consist of the symbol for the element in the... Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep The molecular orbital diagram representing this order of energy levels is shown in fig. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value is negative or zero, it means that the molecule is unstable. Molecular Orbital diagram of NO(nitric oxide) molecule Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12.
Molecular Orbital Theory: Energy level diagram for molecular orbitals Molecular orbital theory was put forward by Hund and Mullikan in 1932. This theory is modern and more rational. This theory assume that in molecules, atomic orbitals lose their identity and the electrons in molecules are present in new orbitals called molecular orbitals.
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here. However, with more atoms, computers are required to calculate how the atomic orbitals combine.
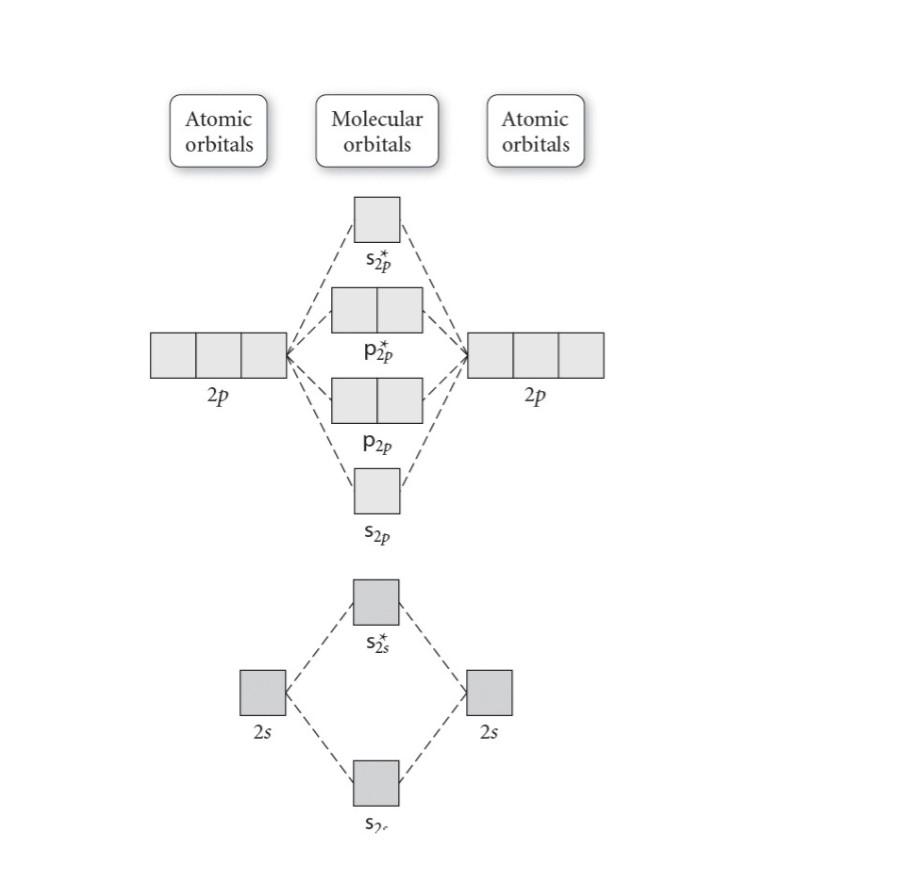
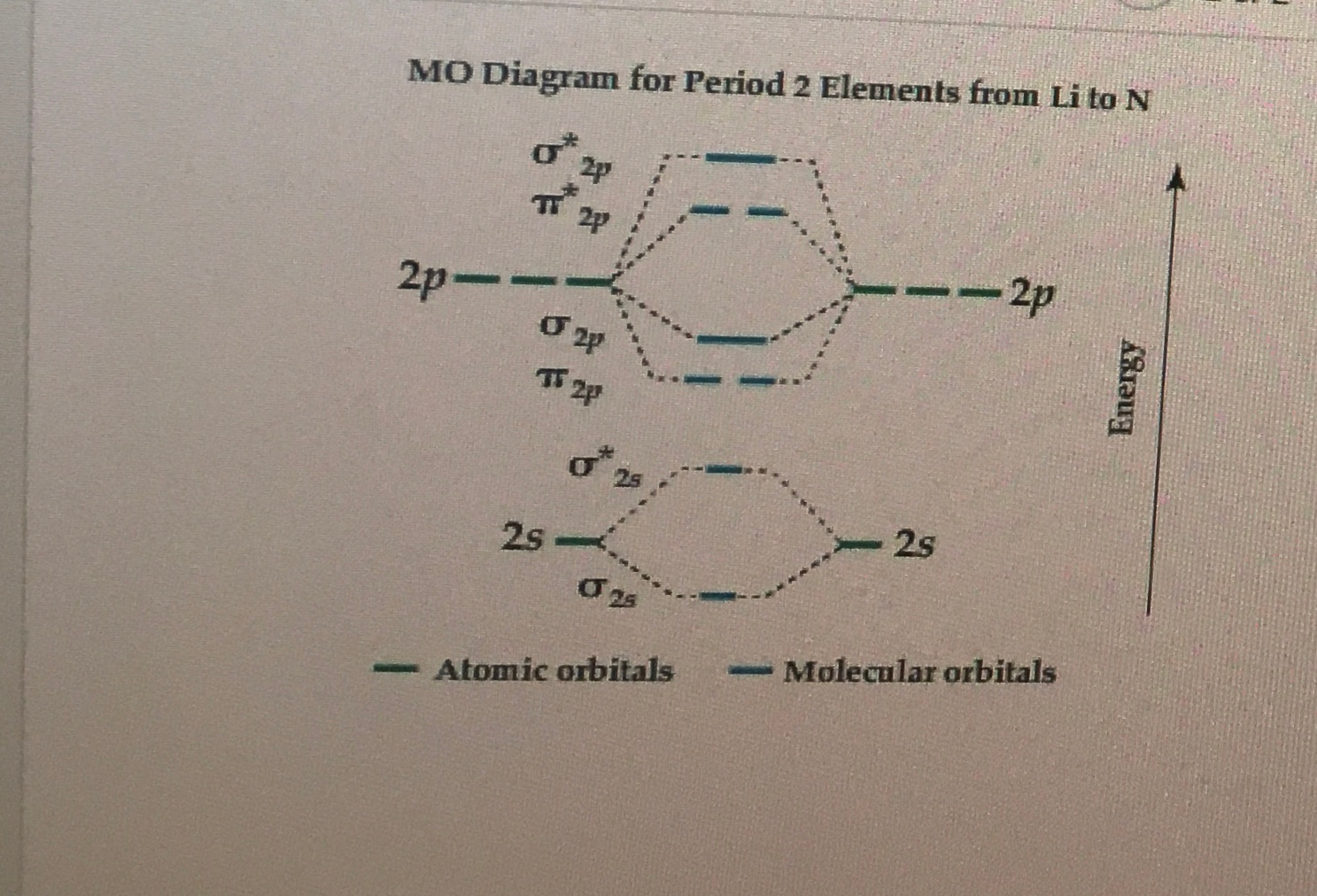
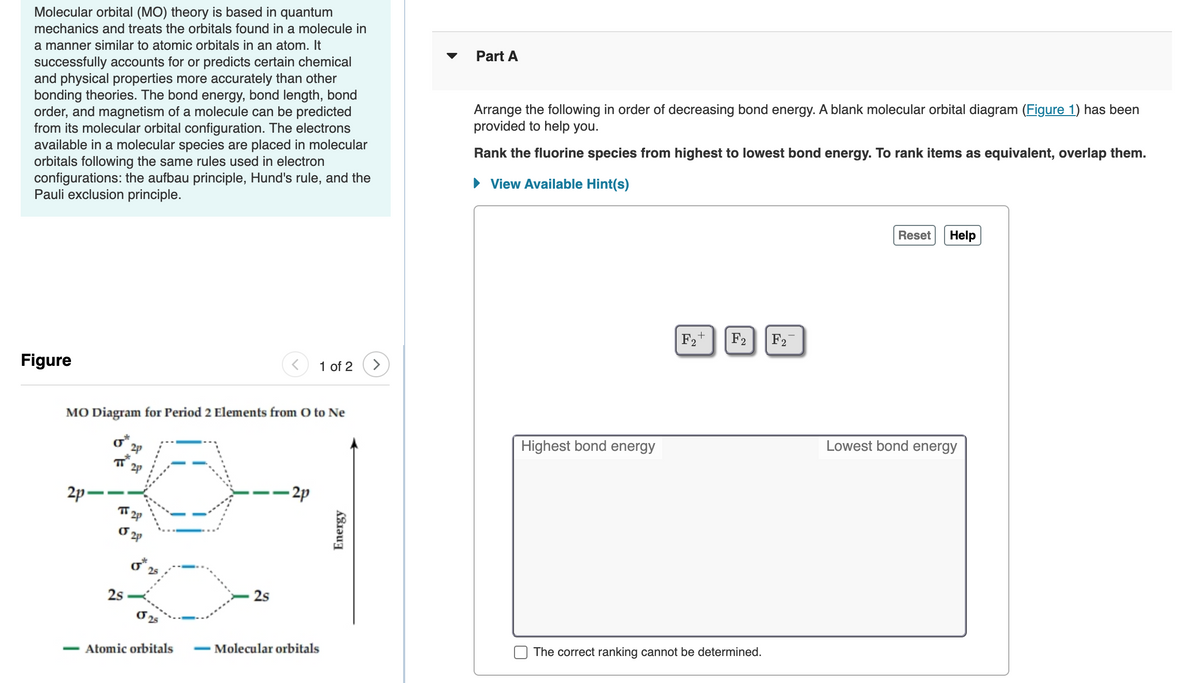
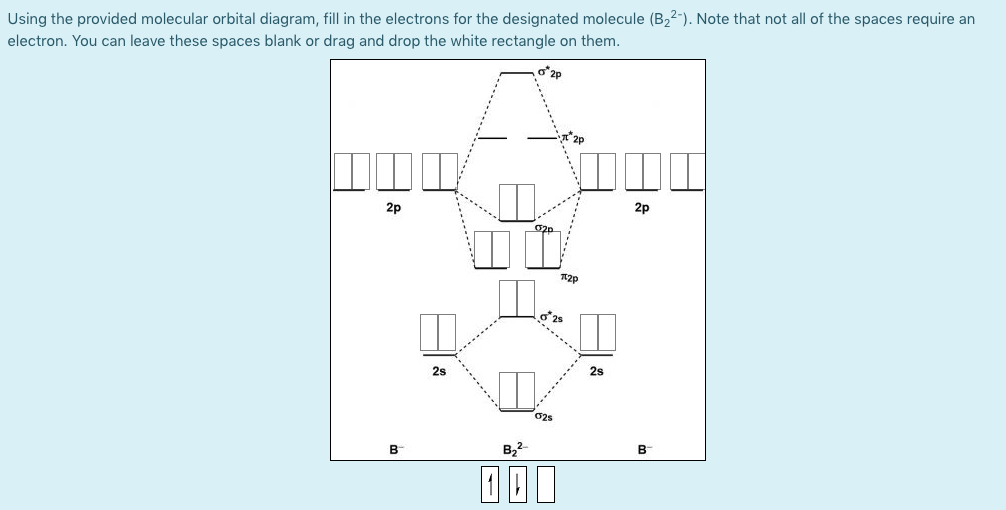
Solved Molecular Orbital Diagrams and Bond Order... | Chegg.com Transcribed image text : Molecular Orbital Diagrams and Bond Order Constants Periodic Table Part A The blank molecular orbital diagram shown here (Figure 1) applies to the valence of diatomic lithium, beryllium, boron carbon, or nitrogen.
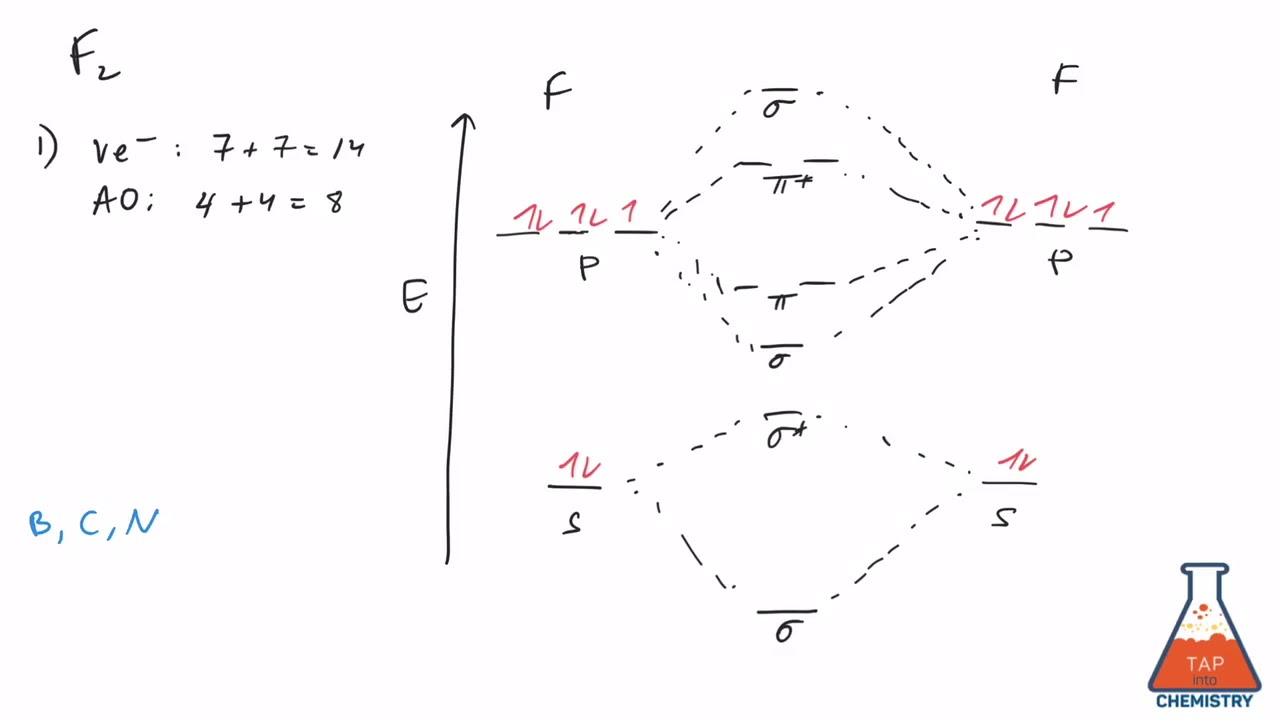
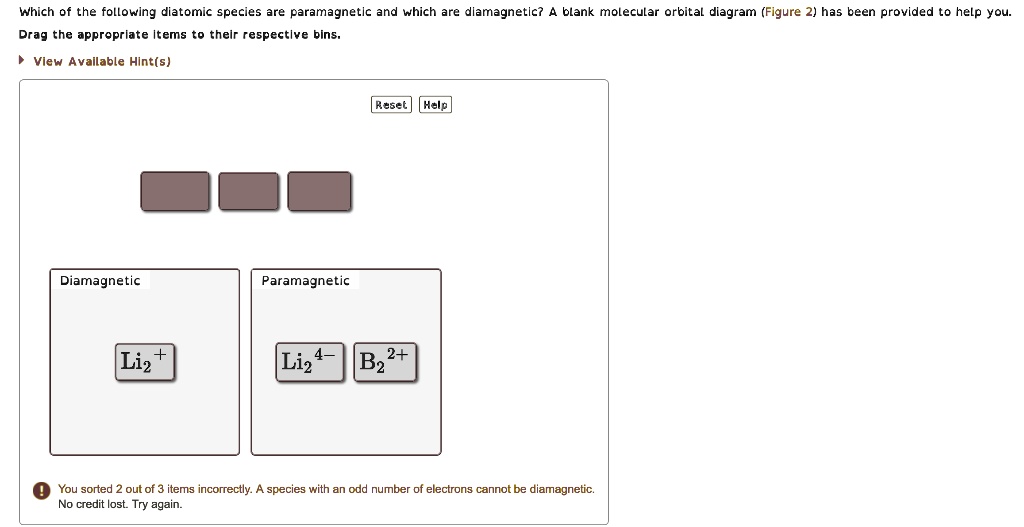
Arrange the following in order of decreasing stability. A blank... A blank molecular orbital diagram (Part A 1 figure) has been provided to help you. Rank the fluorine species from most to least stable. I have no idea what the diagram looks like. BUT wouldn't you expect the F2 atom to be MUCH more stable than either of the others.
8.4 Molecular Orbital Theory - Chemistry The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. The molecular orbital energy diagram predicts that He2 will not be a stable molecule, since it has equal numbers of bonding and antibonding electrons.
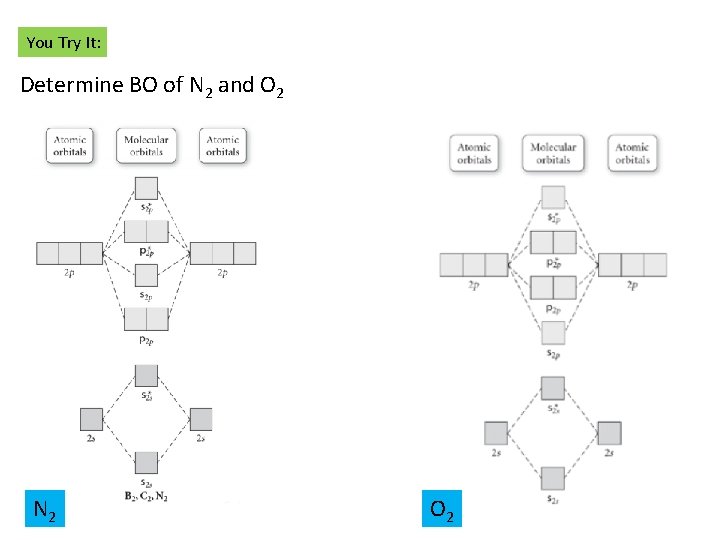
Asked for: molecular orbital energy-level diagram, valence electron... A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. We can therefore use a molecular orbital energy-level diagram and the calculated bond order to predict the relative stability of species such as H2+.
Which software to use to output molecular orbital diagrams? I want to output a molecular orbital diagram. Can anyone recommend a software to do this? Also I searched for a python module, but didn't found a pure solution.
Molecular orbital diagrams - Overleaf, Online LaTeX Editor Molecular orbital diagrams provide qualitative information about the structure and stability of the electrons in a molecule. This article explains how to create molecular orbital diagrams in LaTeX by means of the package MOdiagram. For information about the more traditional molecular structure...
Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory. Valence Bond Theory proposes that electrons are localized between two atoms.
Arrange the following in order of decreasing stability. A blank... A blank molecular orbital diagram (Part A 1 figure) has been provided to help you.Rank the fluorine species from most to least stable. To rank items as equivalent, overlap them.
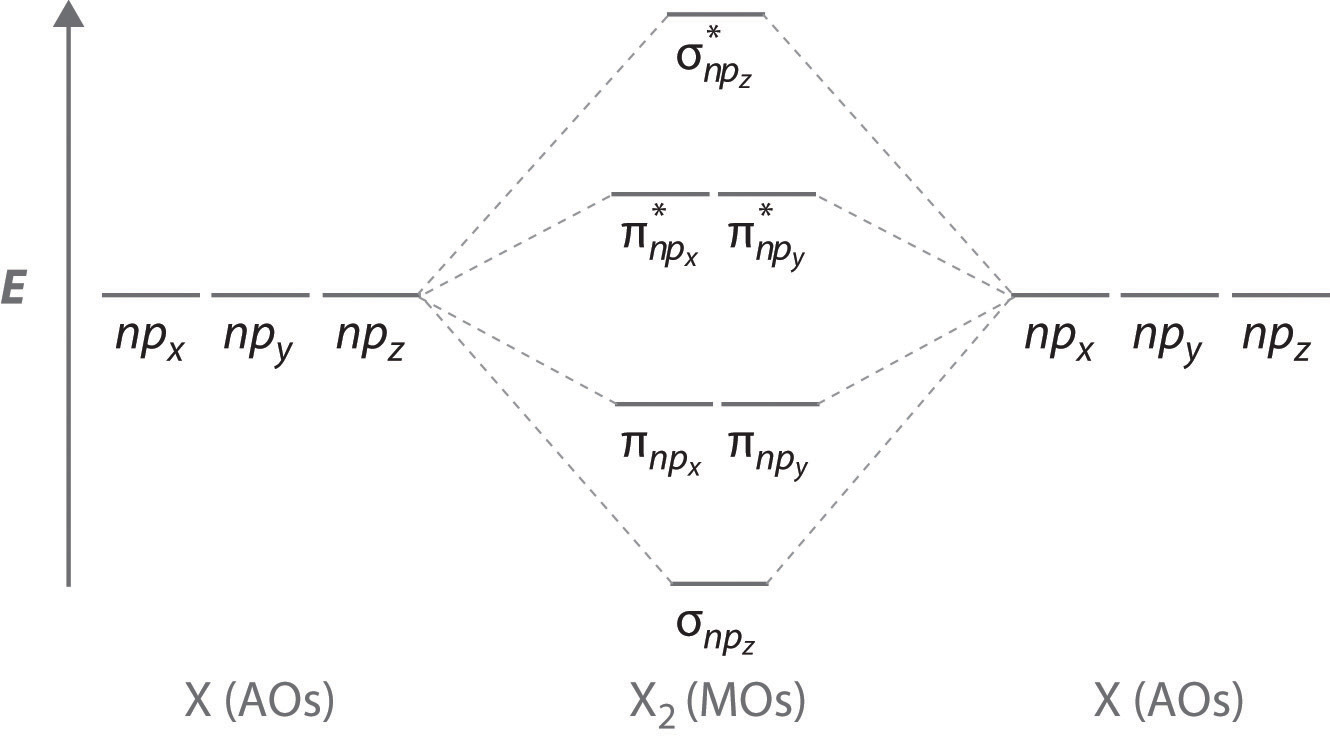
PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Accordingly, a molecular orbital diagram such as Figure 9-5 is inappropriate for heteronuclear diatomic molecules. If the two elements are similar (as in NO or CN mole-cules, for example), we can modify the diagram of Figure 9-5 by skewing it slightly.
Molecular Orbital Theory - Chemistry | Socratic Molecular orbital theory is a method for determining molecular structure. It describes electrons as moving under the influence of the nucleus and not Molecular Orbital (MO) theory better explains the properties of more complex molecules. MO theory explains the partial bonds of NO₃⁻ without using...
Orbital Diagrams and Electron Configuration - Basic Introduction... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram...
Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the...
Structural Biochemistry/How to Construct Molecular Orbital Diagram... 1. First, determine the point group of the molecule. 2. Draw out the orbitals of the central atoms. 3. Determine the irreducible representation of the orbitals of the central atoms. 4. Draw out the possible bonding interaction of the orbitals from the peripheral atoms.












0 Response to "42 blank molecular orbital diagram"
Post a Comment