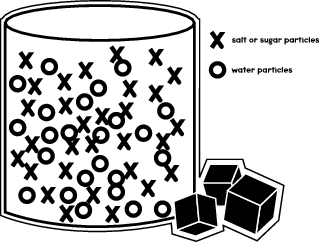
41 diagram of salt dissolving in water
Salinity - Wikipedia Salinity (/səˈlɪnɪti/) is the saltiness or amount of salt dissolved in a body of water, called saline water (see also soil salinity). This is usually measured in. (note that this is technically dimensionless). Volume change on dissolving salt in water | IOPSpark When sodium chloride dissolves in water to make a saturated solution there is a 2.5 per cent reduction in volume. The solubility of salt does not change much with temperature, so there is little profit in using hot water. The salt should be in small crystals and not in rocks or very fine powder.
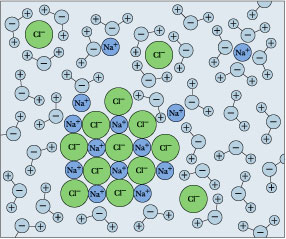
Molecular dynamics of salt dissolving in water - YouTube : Molecular dynamics simulation of salt dissolving in water. The chlorine ions are shown in yellow, sodium in blue. The large chlorine...
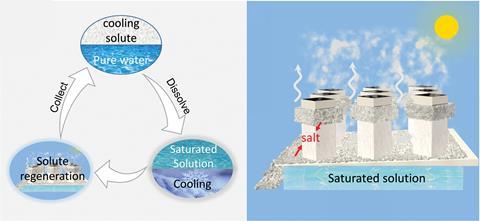
Diagram of salt dissolving in water
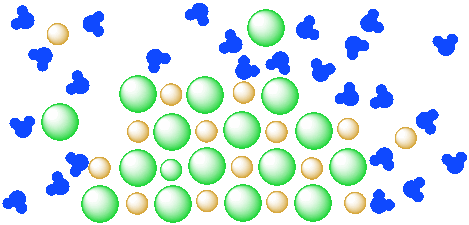
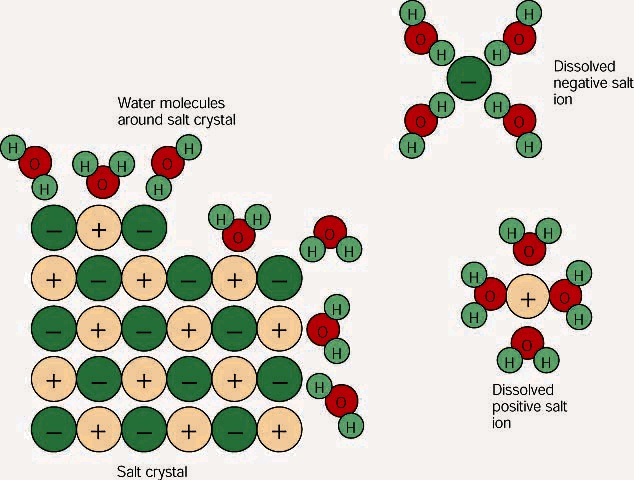
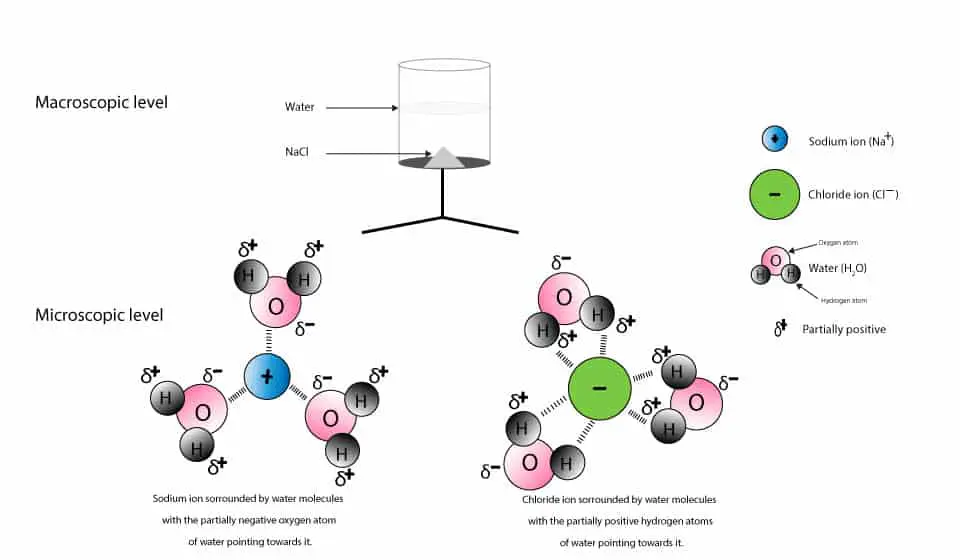
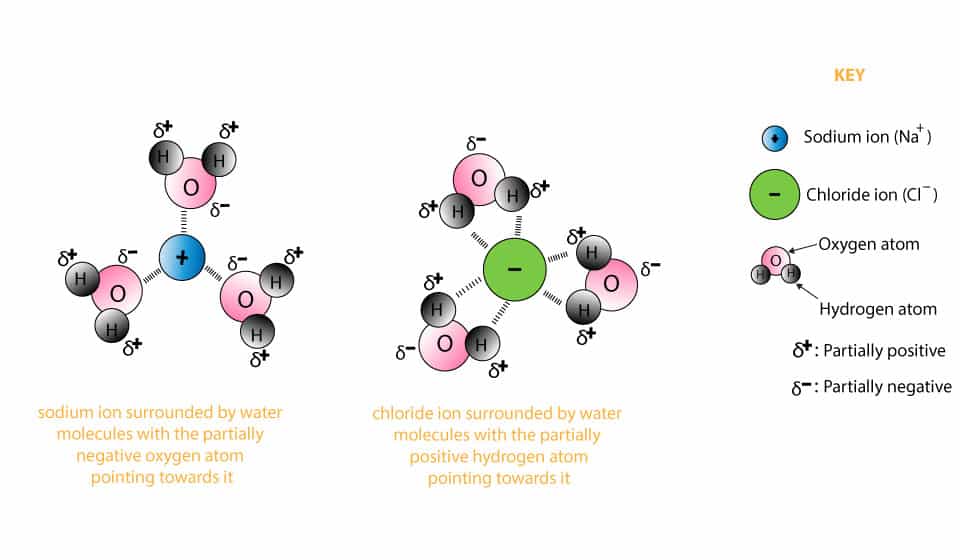
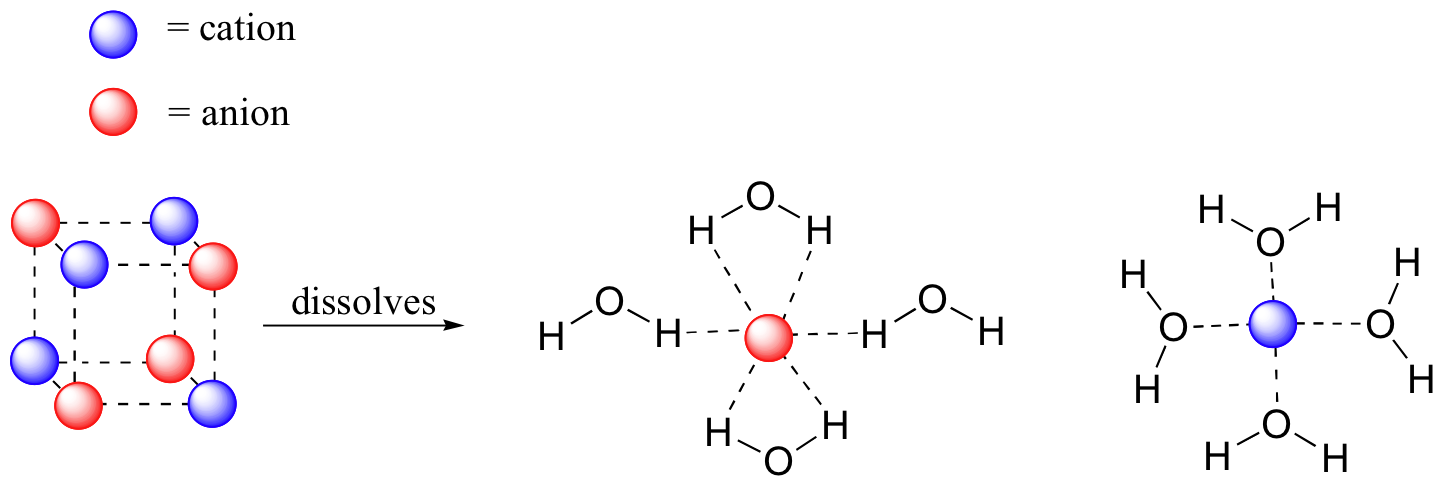
solubility - how dissolving occurs SOLUBILITY - HOW DISSOLVING OCCURS NaCl & H2O: SALT DISSOLVING IN WATER the diagram above shows how water molecules ( red = oxygen + 2 white = hydrogen) are pulling chlorine atoms (green) and sodium atoms (blue above, purple below) away from each other... Dissolving Salt in Water Dissolving Salt in Water. Just bring out a tray of interesting materials and children are excited to get busy. What about using warm water vs. cool water? Dissolving Salt in Water was super fun and engaging. You can try dissolving a variety of solids, like sugar, coffee, sprinkles, flour and more. Water molecules and their interaction with salt | U.S. Geological Survey At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows.
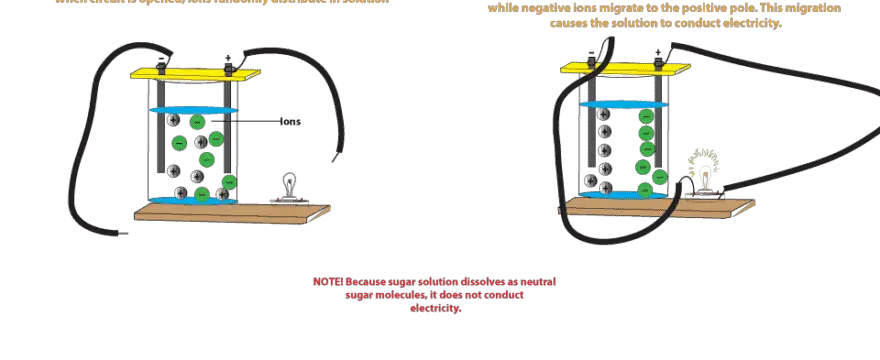
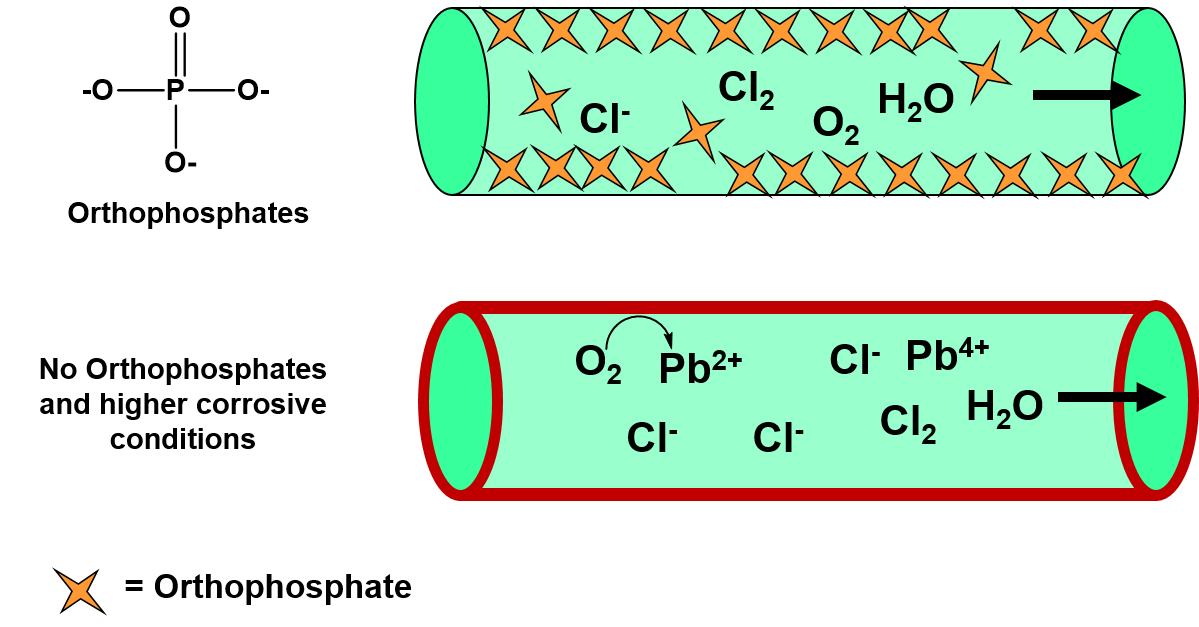
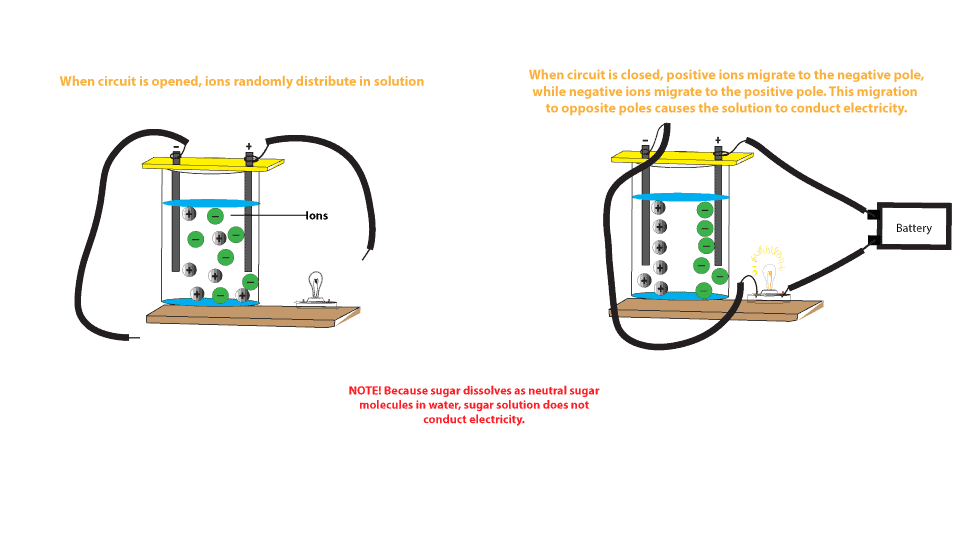
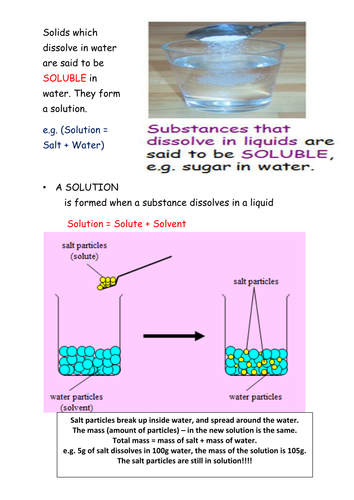
Diagram of salt dissolving in water. Which Solids Dissolve In Water - Cool Science for Kids Things like salt, sugar and coffee dissolve in water. They are soluble. They usually dissolve faster and better in warm or hot water. Solids dissolve faster in hot water as in hot water the water molecules are moving faster, so bump into the solid more often which increases the rate of reaction. Conductivity, Salinity & Total Dissolved Solids - Environmental... Salts dissolve in water to produce an anion and a cation. These ions make up the basis of conductivity in water. Ions conduct electricity due to their positive and negative charges 1. When electrolytes dissolve in water, they split into positively charged (cation) and negatively charged (anion) particles. Dissolved Salt - an overview | ScienceDirect Topics The presence of dissolved salts in the water will accelerate the corrosion process by the provision of ions available Salts dissolved in an aqueous fraction associated with the organic liquid. For instance in crude oil processing steps are The Pd-Mn phase diagram is complex; well defined compounds... PDF Properties of solutions | Annex 1. Salt water solutions • Solids dissolved in water: the salt-water and the sugar-water systems are chosen, as the most proximate to everybody's experience. 1. The interest is just on liquid solutions, since the components do not mix in the solid state, and the amount of salt vapours can be neglected below say 1000 ºC.
Why Does Water Dissolve Salt? | Chapter... | Middle School Chemistry The polarity of water molecules enables water to dissolve many ionically bonded substances. Salt (sodium chloride) is made from positive sodium ions bonded to negative The amount of a substance that can dissolve in a liquid (at a particular temperature) is called the solubility of the substance. Solubility | Why Do Some Solids Dissolve in Water? When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these C12H22O11 molecules are released into solution. The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. Desalting - PetroWiki Most produced water contains salts that can cause problems in production and refining, when solids precipitate to form scale on process equipment. The salts also accelerate corrosion in piping and equipment. Salinity of Water | Dissolved Salts in Sea Water Salinity is the saltiness or measure of dissolved salt in water. 35 g dissolved salt / kg sea water = 35 ppt = 35 o/oo = 3.5% = 35000 ppm = 35000 mg/l. fresh water - official salt concentration limits in drinking water US: 1000 ppm. typical limit agriculture irrigation : 2000 ppm.
Composition of seawater Salinity: the main salt ions making the sea salty. Density: the density of sea water depends on Dissolved gases in seawater The gases dissolved in sea water are in constant equilibrium with the In this diagram one can see how light penetrates no deeper than 150m for photosynthesis. Dissolving Salt in Water However, salt in cold water does not dissolve as well as if the water is warm. They will pour half of the solution in another jar and place one end of the cotton string (mop string) in each of the two jars with Epsom salt solution, as shown in the diagram on the right. Dissolving and Back Again - American Chemical Society Students dissolve salt in water and allow the water to evaporate to investigate the question: What process causes salt to dissolve in water and After viewing a model of salt, students help develop models for the processes of salt dissolving, water evaporating to form a gas, and salt re-forming as... Salty Science: How to Separate Soluble Solutions - Scientific American Salt is soluble in water whereas sand is insoluble (not dissolvable ) in water. Because of this, when the boiling water was added to the mixture of salt and sand, the salt should have dissolved, or disappeared, whereas the sand stayed visible, creating a dark brown solution with possibly some...
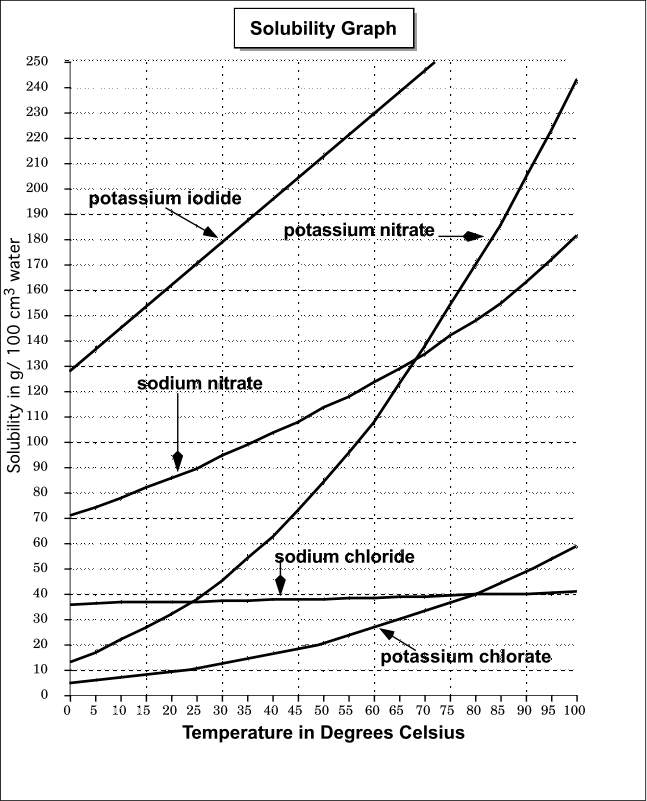
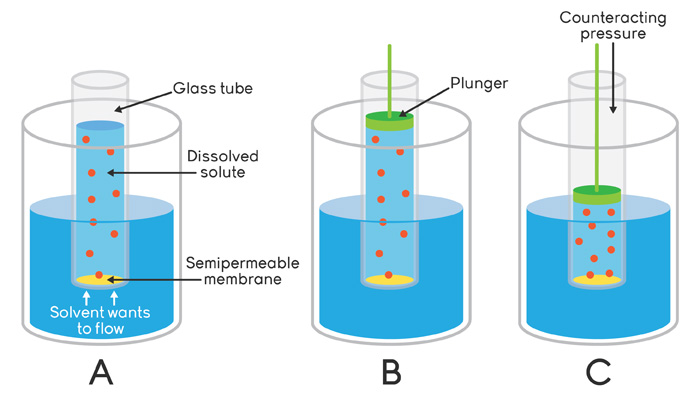
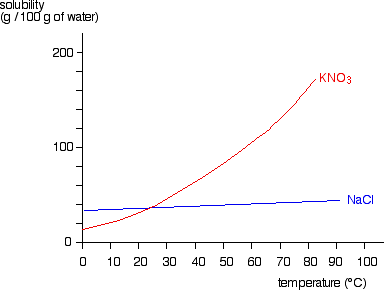
Liquid-Solid Phase Diagrams: Salt Solutions - Chemistry LibreTexts This page looks at the phase diagram for mixtures of salt and water - how the diagram is built up, and how to interpret it. It includes a brief discussion of For example, suppose you have a near-boiling solution of potassium nitrate in water. We'll take a solution containing 100 g of potassium nitrate and...
When common salt is dissolved in water | Toppr Ask A solution of this salt was prepared by dissolving 0.25g of this sodium salt of protein in 10g of water and ebulliscopic analysis revealed that solution boils at temperature 5.93×10−3∘C higher than the normal boiling point of pure water. Kb of water 0.52kgmol−1.
Dissolving Salt in Water: Chemical or Physical Change? Therefore, dissolving salt in water is a chemical change. The reactant (sodium chloride, or NaCl) is different from the products (sodium cation and Thus, any ionic compound that is soluble in water would experience a chemical change. In contrast, dissolving a covalent compound like sugar does...
Salts/Salt Mixtures - Saltwiki Author: Amelie Stahlbuhk back to Saltwiki:Community portal. The key properties of salts and salt mixtures are described by means of phase and solubility diagrams. Mainly those salts relevant to cultural heritage objects are addressed.
How to Dissolve Salt in Water: 9 Steps (with Pictures) - wikiHow Warmer water will dissolve more salt than cooler water, regardless of the type of salt you are using. If you are doing a formal experiment, you The amount of salt you can dissolve in water depends on a few variables like temperature and purity. To dissolve salt into water, just stir it in with a spoon or...
Salinity - Dissolved Salts, Measuring Salinity - Windows to the Universe Ocean water is about 3.5% salt. That means that if the oceans dried up completely, enough salt would be left behind to build a 180-mile-tall, one About 90 percent of that salt would be sodium chloride, or ordinary table salt. Chlorine, sodium and the other major dissolved salts of the ocean are listed in...
How Does Salt Dissolve in Water? Water dissolves salt by dissociating the ions in salt from each other. Because water is a polar molecule, each of its ends holds Although common table salt easily dissolves in water, not all ionic salts do. If the strength of the attraction between the ions is much greater than the strength exerted...
Why is dissolving salt in water an endothermic process? - Quora For instance, dissolving calcium salts in water is typically very exothermic because the calcium ion is relatively small and has a charge of +2, enabling very, very (However, there are always exceptions - NaCl's dissolution in water is endothermic. Not all metals form salts whose dissolution is exothermic.
PDF CFD Modelling of dissolved salt / water flow Water dissolved salts forms a great fouling resistance to heat transfer cases, reduce operating pressure and partially blocks flow Adopting two-resistance model for the two-phase heat transfer exchange Dissolved salts is considered as a particle transport solid having the same flow travel speed.
calculus - A spherical ball of salt is dissolving in water - Mathematics... Connect and share knowledge within a single location that is structured and easy to search. Learn more. A spherical ball of salt is dissolving in $\begingroup$ Trick question... the NaCl crystals are a cubic shape. So there cannot be a truly spherical ball of salt, only a quantized approximation...
What Dissolves Salt Besides Water? | Sciencing Table salt is just one type of salt and is water soluble. Other water-soluble salts include nitrates, chlorides, and sulfates. There are exceptions to the rule, though. A salt is considered insoluble if, by Purdue University's definition, it can dissolve in room temperature water to a concentration of...
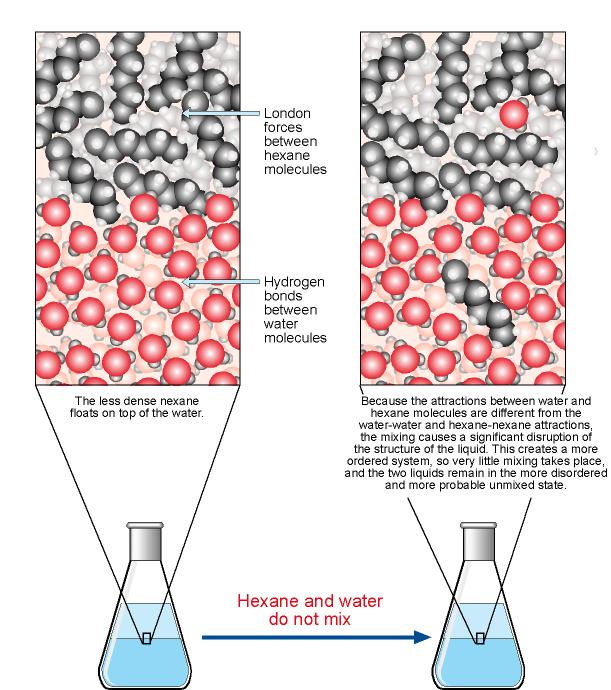
Why Does Salt Dissolve in Water But Not Oil? | LEAFtv Why Salt Dissolves in Water. Salt or sodium chloride consists of sodium and chloride ions joined by an ionic bond to form a charged NaCl molecule. Why Salt Does Not Dissolve in Oil. Oil molecules do not contain any charge. Oil is comprised of long chains of hydrogen and carbon atoms linked to each...
Water molecules and their interaction with salt | U.S. Geological Survey At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows.
Dissolving Salt in Water Dissolving Salt in Water. Just bring out a tray of interesting materials and children are excited to get busy. What about using warm water vs. cool water? Dissolving Salt in Water was super fun and engaging. You can try dissolving a variety of solids, like sugar, coffee, sprinkles, flour and more.
solubility - how dissolving occurs SOLUBILITY - HOW DISSOLVING OCCURS NaCl & H2O: SALT DISSOLVING IN WATER the diagram above shows how water molecules ( red = oxygen + 2 white = hydrogen) are pulling chlorine atoms (green) and sodium atoms (blue above, purple below) away from each other...















0 Response to "41 diagram of salt dissolving in water"
Post a Comment