40 orbital filling diagram for nitrogen
Orbital Filling Diagram For Nitrogen - schematron.org Feb 09, 2018 · 02.09.201802.09.20186 Commentson Orbital Filling Diagram For Nitrogen Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s. Nitrogen (N) - ChemicalAid Nitrogen (N) has an atomic mass of 7. Find out about its chemical and physical ... Orbital Diagram. N - Nitrogen - Orbital Diagram - Electron Configuration ...
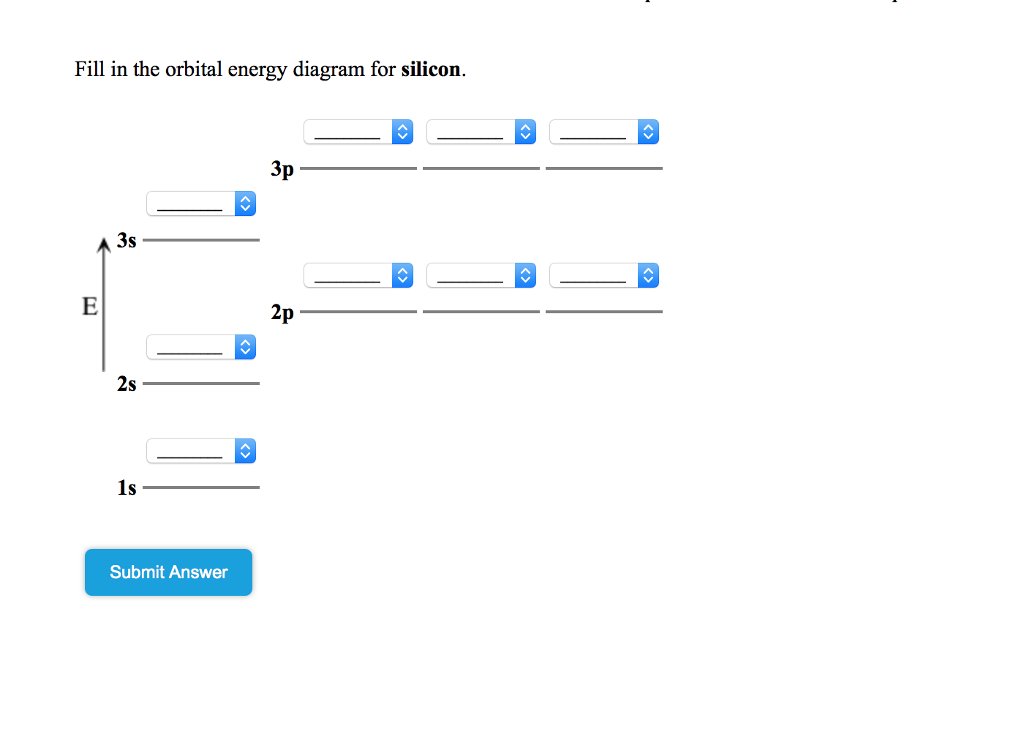
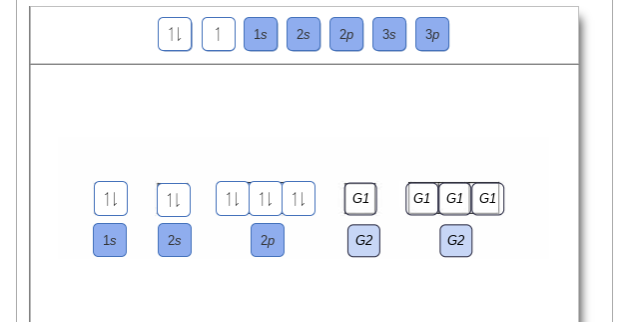
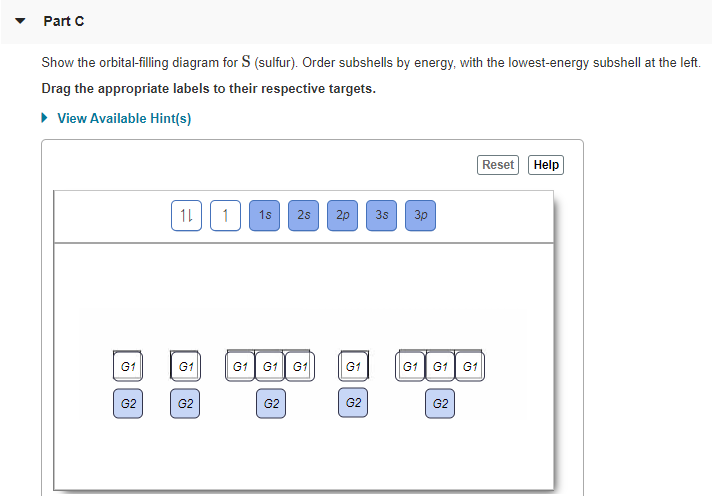
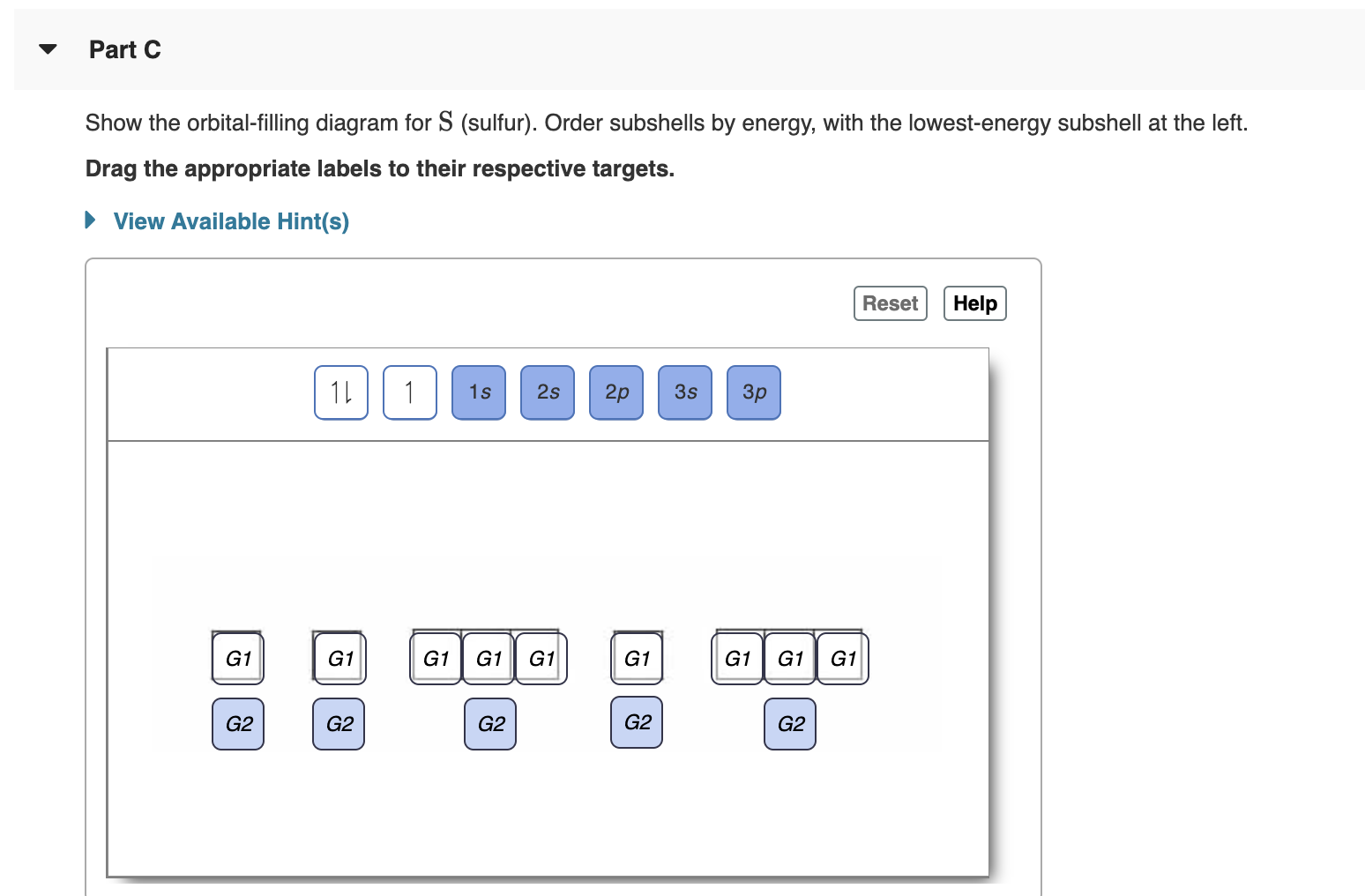
Solved Show the orbital-filling diagram for N (nitrogen ... Chemistry questions and answers. Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left Drag the appropriate labels to their respective targets. View Available Hint (s) Reset Help 11 || 1 18 2s 2p 3s ap Group 1 GT G1 G1 G1 GI GT G161 G2 G2 G2 G2 G2.
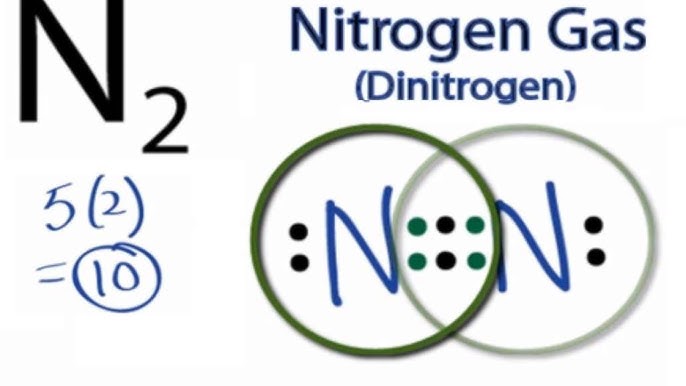
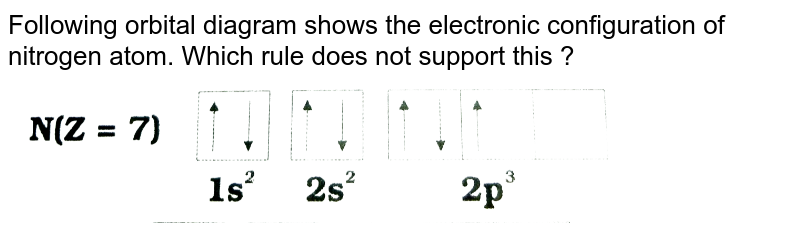
Orbital filling diagram for nitrogen
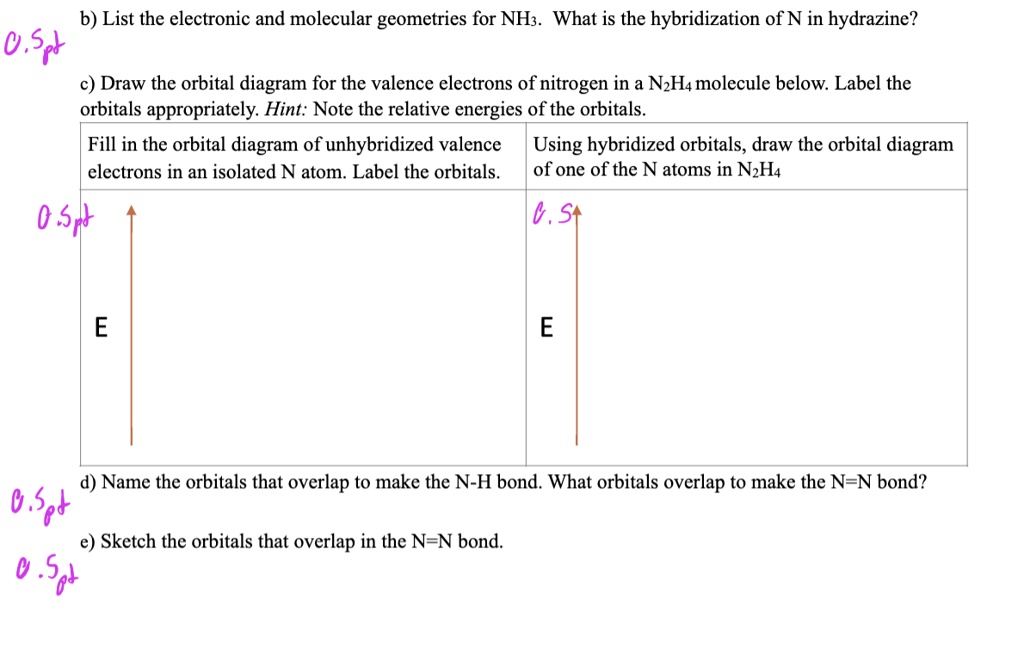
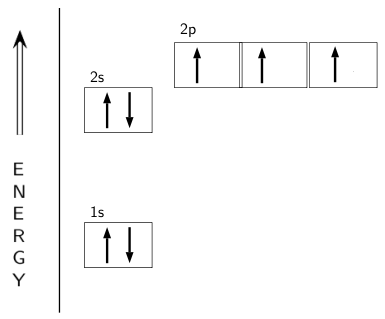
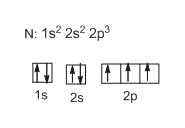
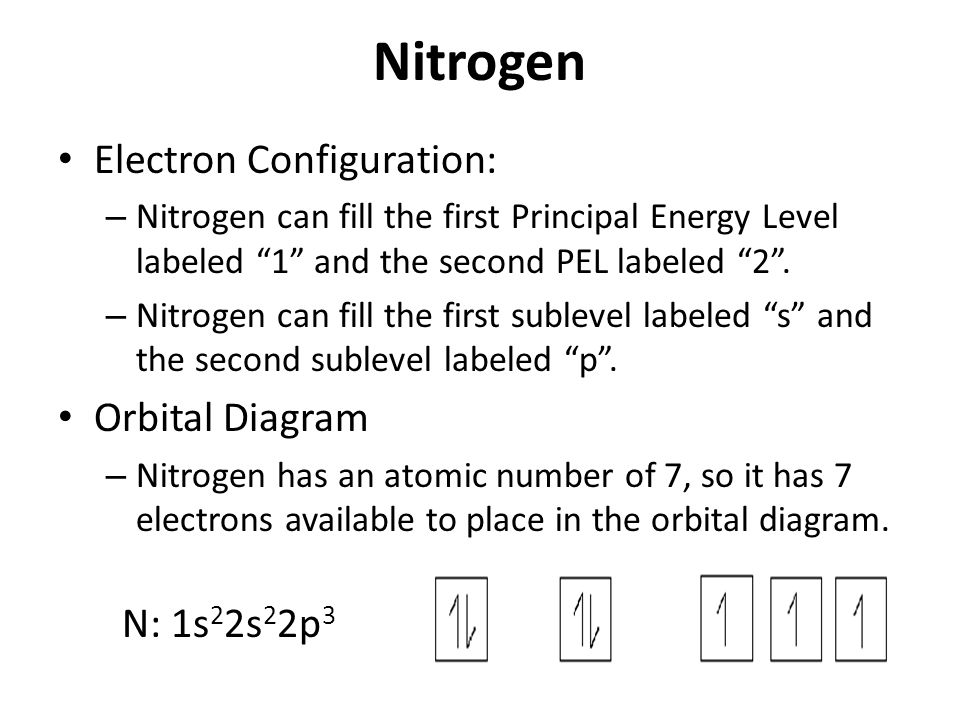
Draw and explain the orbital-filling diagram for nitrogen. The periodic table shows us that nitrogen (N) has an atomic number of 7. As a result, a neutral nitrogen atom will have 7 electrons. In orbital... Nitrogen Orbital diagram, Electron configuration, and ... The orbital diagram for nitrogen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The nitrogen orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, and the rest three electrons in 2p orbital. The orbital diagram for a ground-state electron configuration of nitrogen atom is as follow- Nitrogen Electron Configuration (N) with Orbital Diagram 21 Jan 2021 — When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next ...
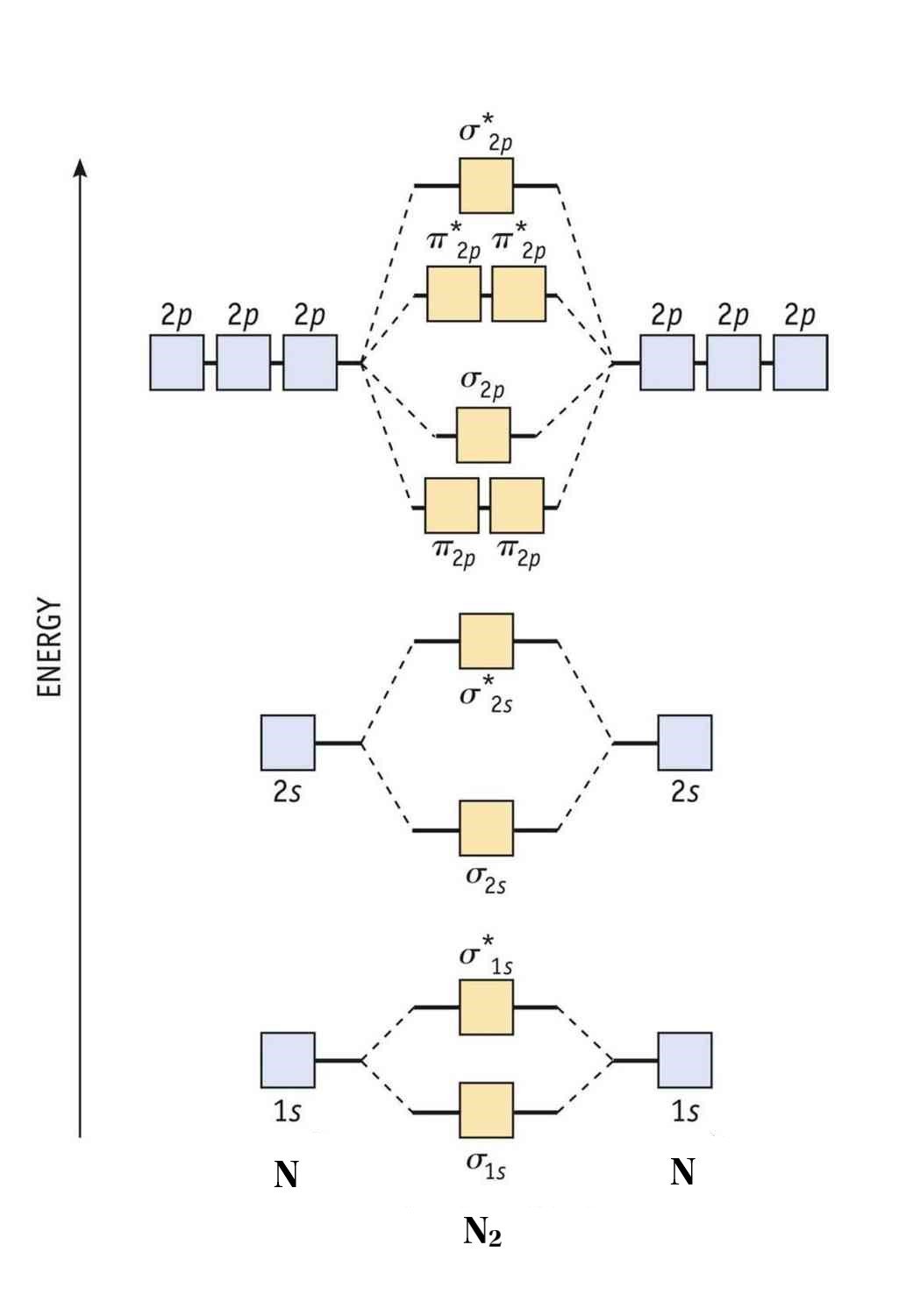
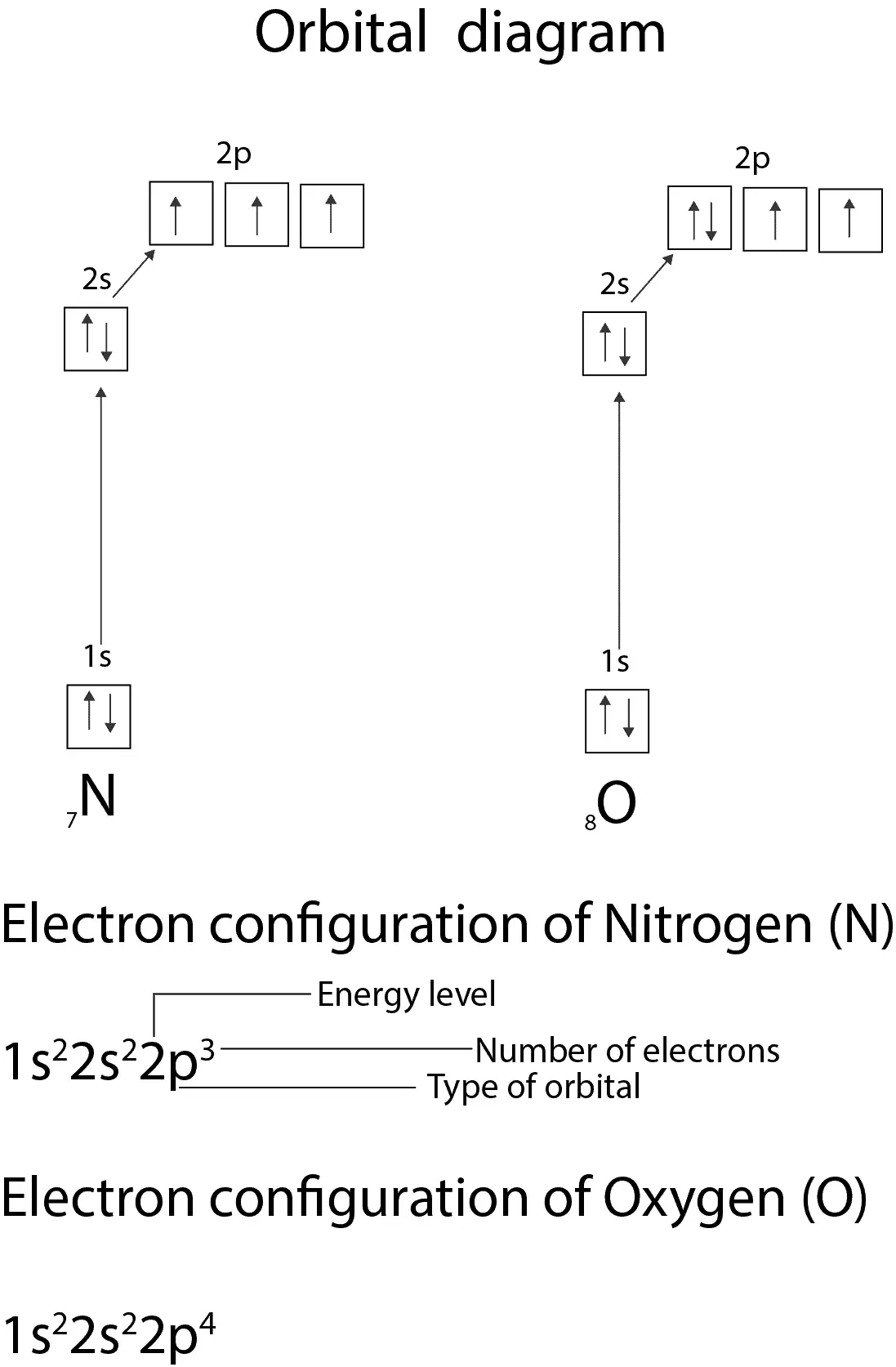
Orbital filling diagram for nitrogen. Nitrogen(N) electron configuration and orbital diagram Nitrogen (N) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund’s principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. Hund's Rule and Orbital Filling Diagrams - Lumen Learning Use orbital filling diagrams to describe the locations of electrons in an atom. ... Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. Orbital Filling Diagram For Nitrogen In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s. Use orbital filling diagrams to describe the locations of electrons in an atom. Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... Feb 15, 2021 · If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital.
Nitrogen Electron Configuration (N) with Orbital Diagram 21 Jan 2021 — When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next ... Nitrogen Orbital diagram, Electron configuration, and ... The orbital diagram for nitrogen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The nitrogen orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, and the rest three electrons in 2p orbital. The orbital diagram for a ground-state electron configuration of nitrogen atom is as follow- Draw and explain the orbital-filling diagram for nitrogen. The periodic table shows us that nitrogen (N) has an atomic number of 7. As a result, a neutral nitrogen atom will have 7 electrons. In orbital...



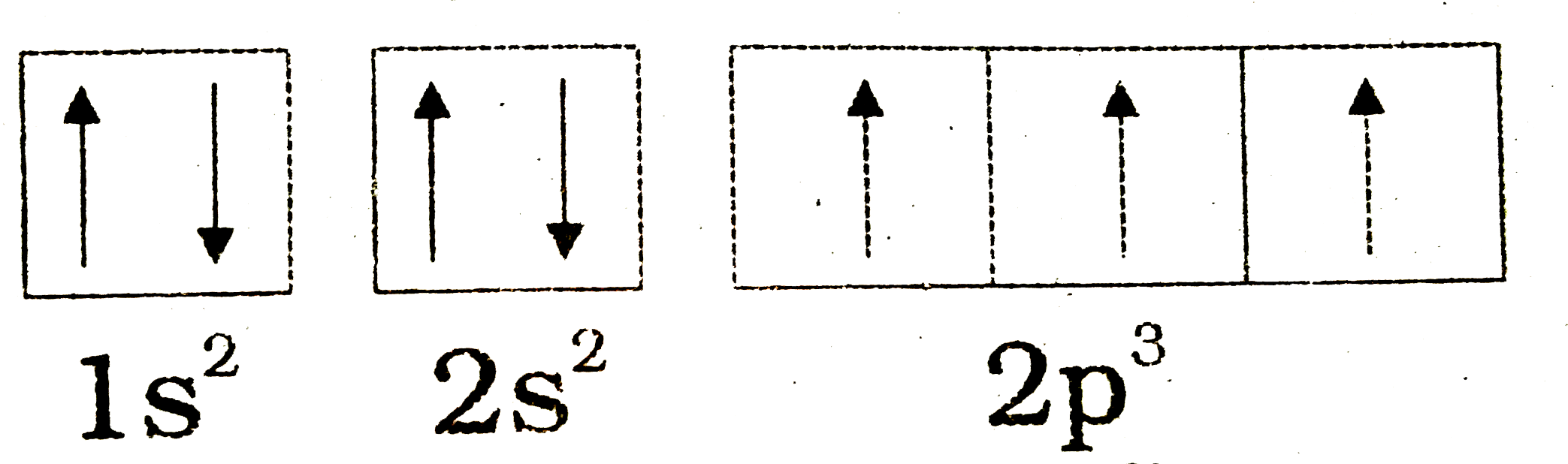



















0 Response to "40 orbital filling diagram for nitrogen"
Post a Comment