40 orbital diagram for ti
Titanium (Ti) - ChemicalAid Titanium (Ti) has an atomic mass of 22. Find out about its chemical and ... Orbital Diagram. Ti - Titanium - Orbital Diagram - Electron Configuration ... Draw and explain the orbital diagram for titanium. | Study.com Titanium is a transition element which has the atomic number 22. It forms various types of compounds like titanium tetrachloride and titanium...
What is the orbital diagram of TI? – AnswersToAll Feb 08, 2010 · What is the correct orbital diagram for phosphorus? The p orbital can hold up to six electrons. We’ll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we’ll move to the 3p where we’ll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s22s22p63s23p3.

Orbital diagram for ti
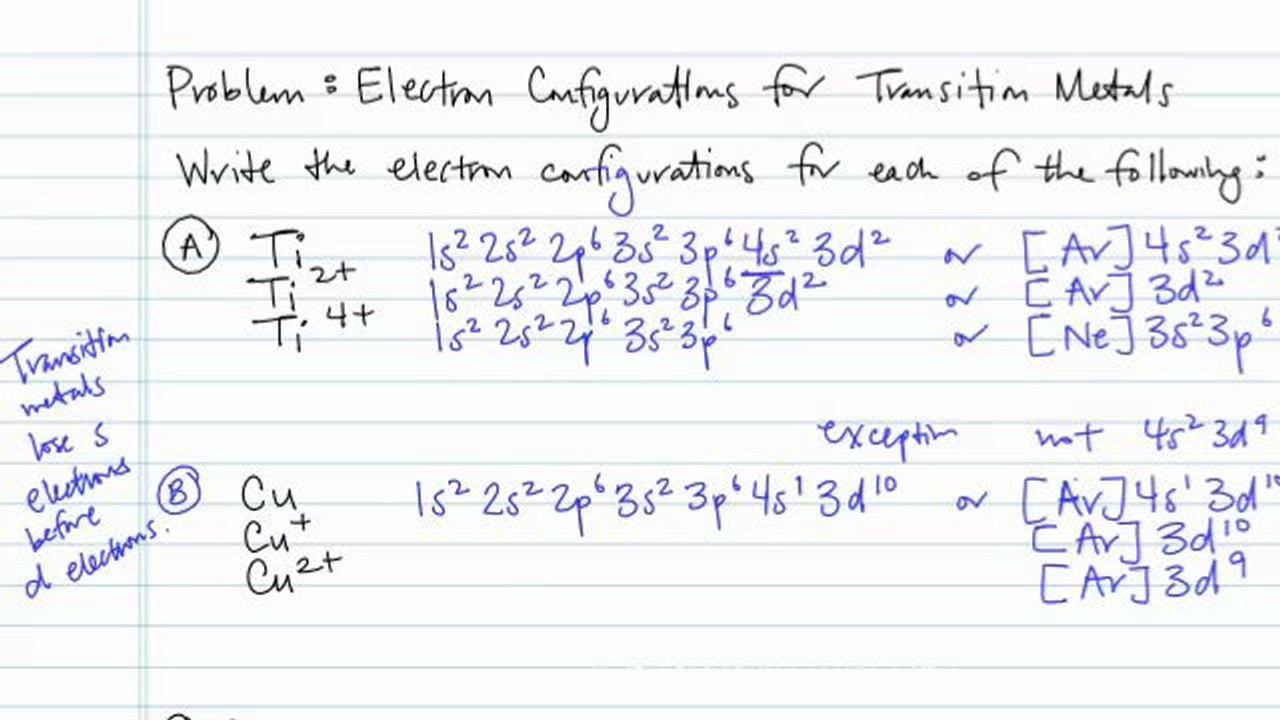
Electron configuration for Titanium (element 22). Orbital diagram Valence electrons of Titanium ; s, 4, 0, 0, -1/2 ; d, 3, 2, -2, +1/2. What is the electron configuration of "Ti"^(2+)? | Socratic Ti2+:[Ar]3d2. Explanation: A good place to start when trying to figure out the electron configuration of an ion is the electron ... Orbital Diagram For Ti2+ - schematron.org Jun 12, 2018 · A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. What is the orbital diagram for Ti 2+?
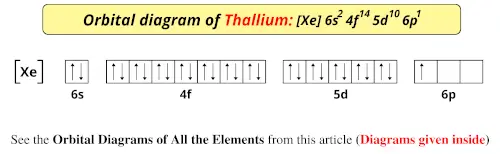
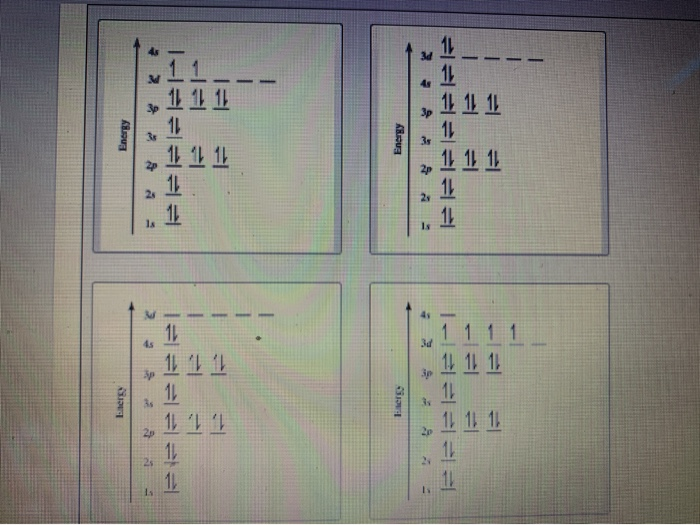
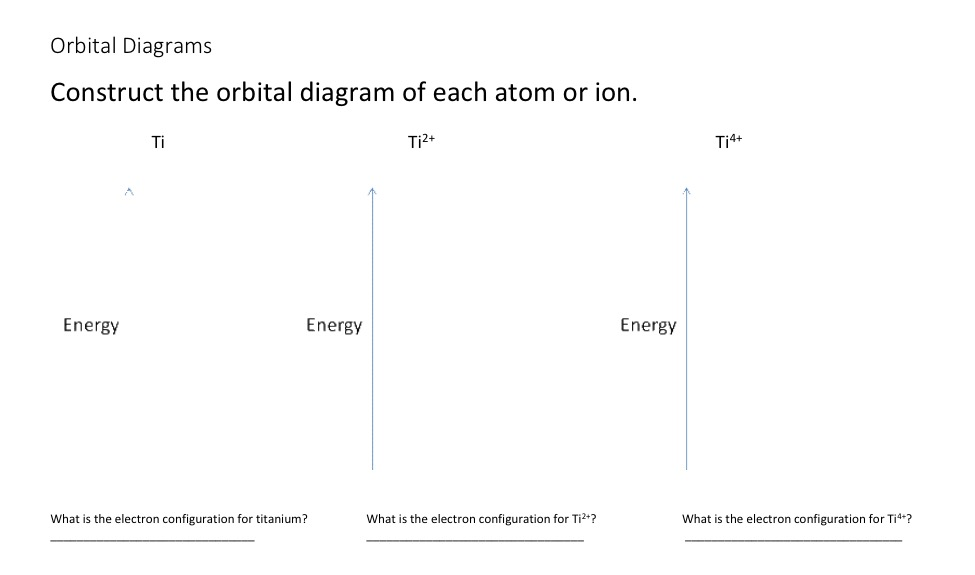
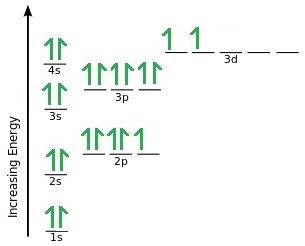
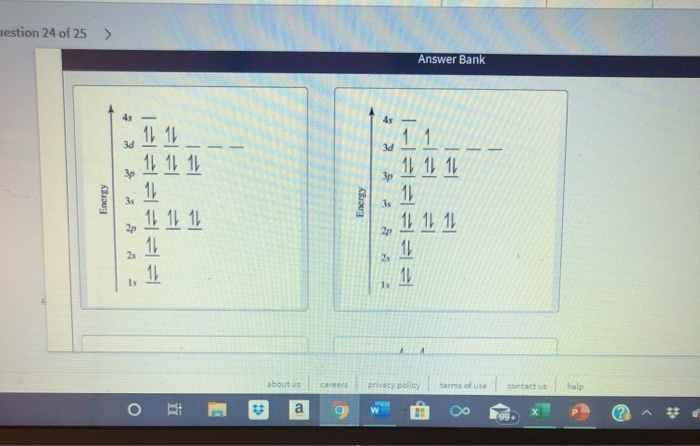
Orbital diagram for ti. Orbital Diagram Of Ti2+ - schematron.org Aug 14, 2018 · Construct the orbital diagram of each atom or ion. Ti. Ti 2+ Ti 4+ Next. Practice Problems. Choose the orbital diagram that represents the gro Consider the portion of the orbital filling diagra Choose the orbital diagram that represents the gro Identify the element which has the following parti. Orbital Diagram of Titanium (Ti), electron configuration, and ... This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble... Titanium(Ti) electron configuration and orbital diagram Titanium (Ti) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund’s principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. Titanium Electron Configuration (Ti) with Orbital Diagram 26 Jan 2021 — Titanium Electron Configuration: Titanium is a chemical element that has a chemical symbol Ti. Its atomic number is 22.
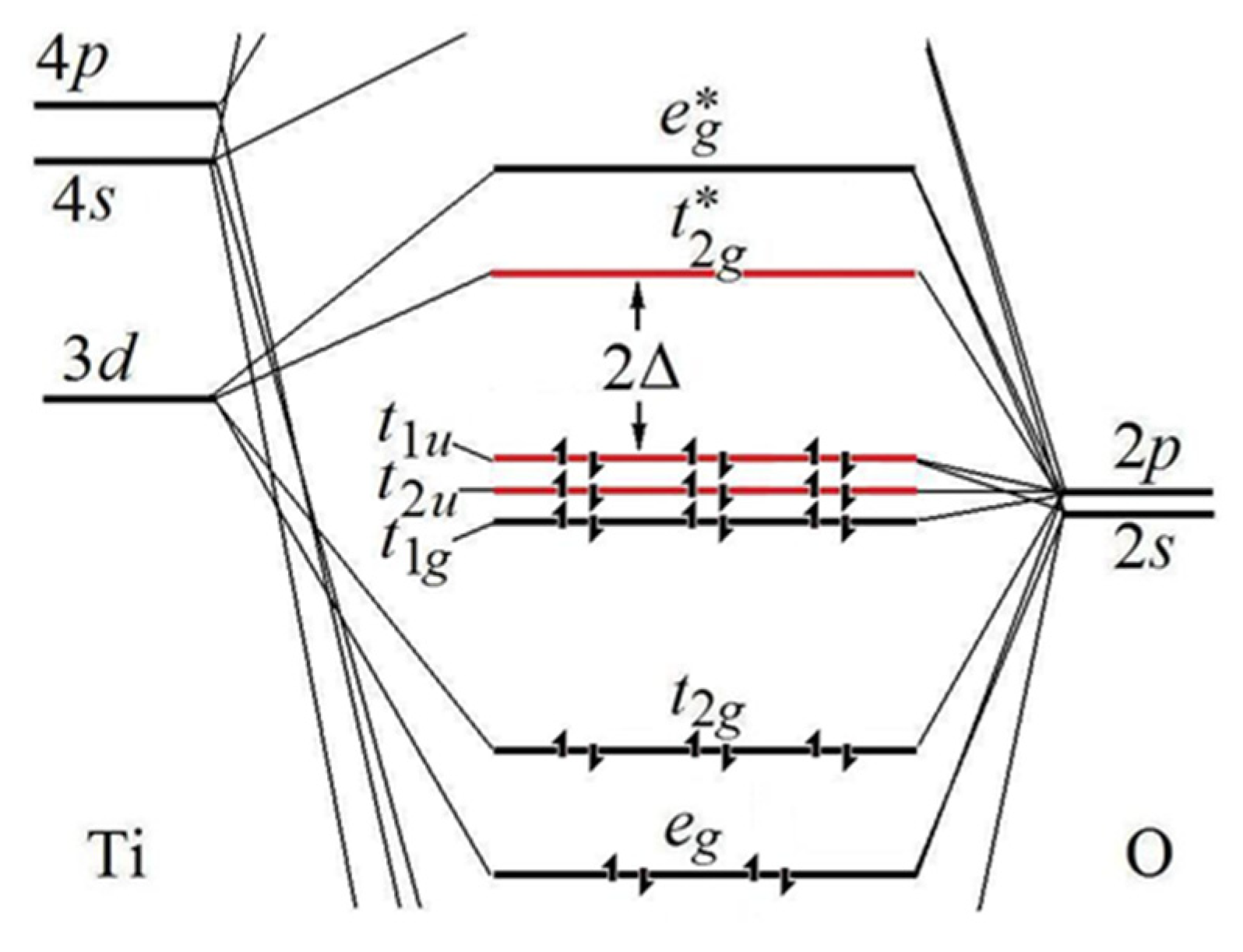
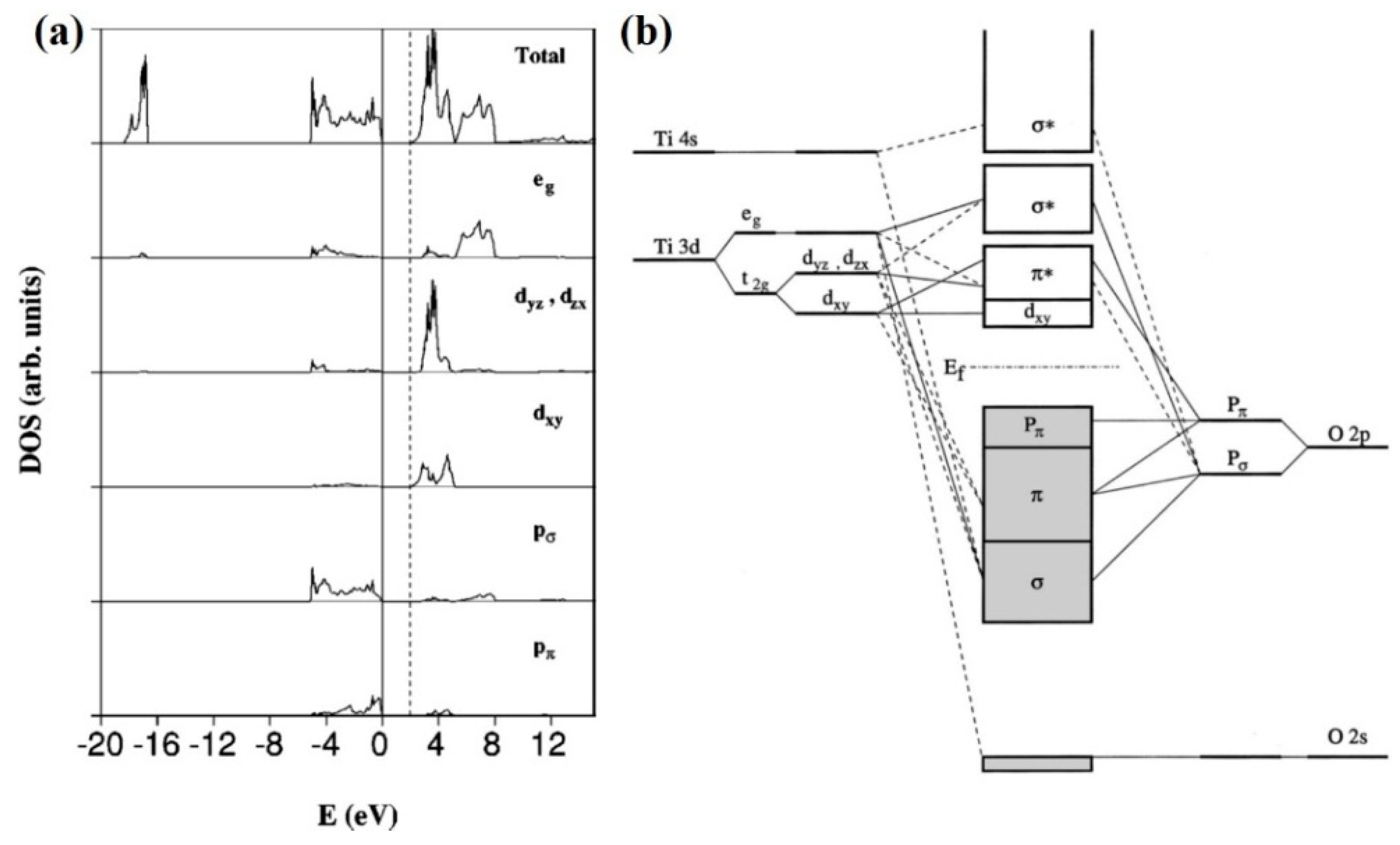
Molecular orbitals diagrams of [Ti(H2O)6]3+ Nov 20, 2021 · Molecular orbitals diagrams of [Ti (H2O)6]3+. 1. M. O. diagram for [Ti (H2O)6]3+ Dr. Mithil Fal Desai Shree Mallikarjun and Shri Chetan Manju Desai College Canacona Goa. 2. t* 1u a1g t2g, eg a1g, t1u, eg a1g t1u a* 1g e* g eg t1u Δo t2g Metal (Ti3+)orbitals Ti3+→ [Ar] 3d1, 4s0 1e- Ligand group (H2O) orbitals 6 x 2 = 12 e- σ [Ti (H2O)6]3 ... Orbital Diagram For Ti2+ - schematron.org Jun 12, 2018 · A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. What is the orbital diagram for Ti 2+? What is the electron configuration of "Ti"^(2+)? | Socratic Ti2+:[Ar]3d2. Explanation: A good place to start when trying to figure out the electron configuration of an ion is the electron ... Electron configuration for Titanium (element 22). Orbital diagram Valence electrons of Titanium ; s, 4, 0, 0, -1/2 ; d, 3, 2, -2, +1/2.



















![MO diagram of [M(CO)8]q (M, q = Ca, 2−; Sc, 1−; Ti, 0; V, 1 ...](https://www.researchgate.net/publication/340232529/figure/fig3/AS:959992203055127@1605891385270/MO-diagram-of-MCO8q-M-qCa-2-Sc-1-Ti-0-V-1-Cr-2-Ba-2-a-The-s.png)


0 Response to "40 orbital diagram for ti"
Post a Comment