38 methane molecular orbital diagram
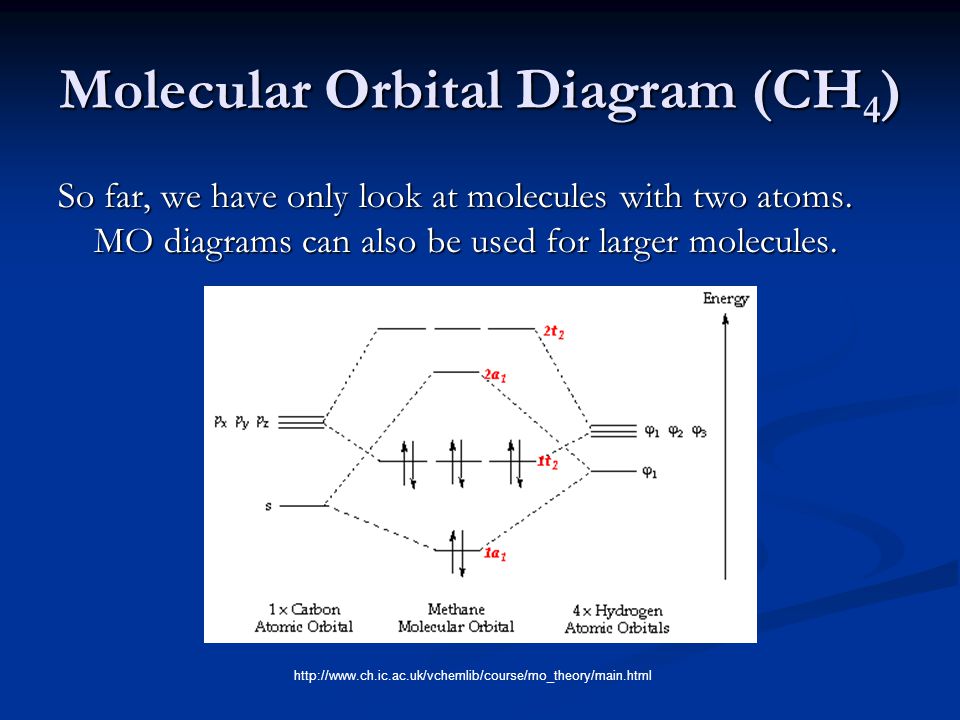
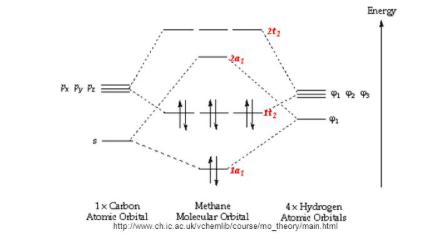
Molecular orbitals of methane: symmetry or hybridization ... The three molecular orbitals (MOs) of methane in the ground electronic state (X 1 A 1 ), namely, the core MO, 1 a1 and the valence MOs, i.e. 2 a1 and three-fold energy degenerate MOs 1 t2, are studied in both coordinate space and momentum space. Molecular orbitals of methane: Symmetry or hybridization ... The methane molecule is the result of the overlap of each sp 3 orbital with a 1s hydrogen orbital. Although the hybridization model is able to explain the molecular geometry, it fails to explain...
Solved PREVIOUS QUESTION 3. Molecular orbital theory ... Molecular orbital theory. Methane (CH4) has tetrahedral geometry and Td point group symmetry. Derive a molecular orbital diagram for methane by performing the following steps: a. Determine the reducible representation (Γ) describing the symmetry of the four H 1s orbitals that are involved in σ bonding with the valence atomic orbitals

Methane molecular orbital diagram
PDF Consider ethylene (also called ethene): C H . Draw a Lewis ... • Example - In methane, CH 4, the sp3 hybrid orbitals point at the 1s hydrogen orbitals, and the atomic orbitals are added and subtracted to create molecular orbitals • The other resulting MO is higher in energy than the two atomic orbitals and is antibonding • Only the lower-energy orbital is populated with electrons in methane PDF Deriving the MO diagram for square planar methane. square planar methane. With no lone pairs, the electronic and molecular geometry are the same. Technique for constructing LGO's: I Draw Lewis structure and assign VSEPR geometry (already done above) II Assign a point group to the molecular geometry III determine the central atom's VB hybrid orbitals for the electronic geometry PDF ORBITALS and MOLECULAR REPRESENTATION Construct the molecular orbital diagram for dichlorine. x y z z y 3 x y z z y 4 Showing the p orbitals. Showing the s and p orbitals. ORBITALS AND MOLECULAR REPRESENTATION 11. CARBON ORBITALS Methane Ethane METHANE AND ETHANE C H H H H CH4 C C H H H H H H C2H6 1 2 Color conventions: Hydrogen atoms are shown in gray.
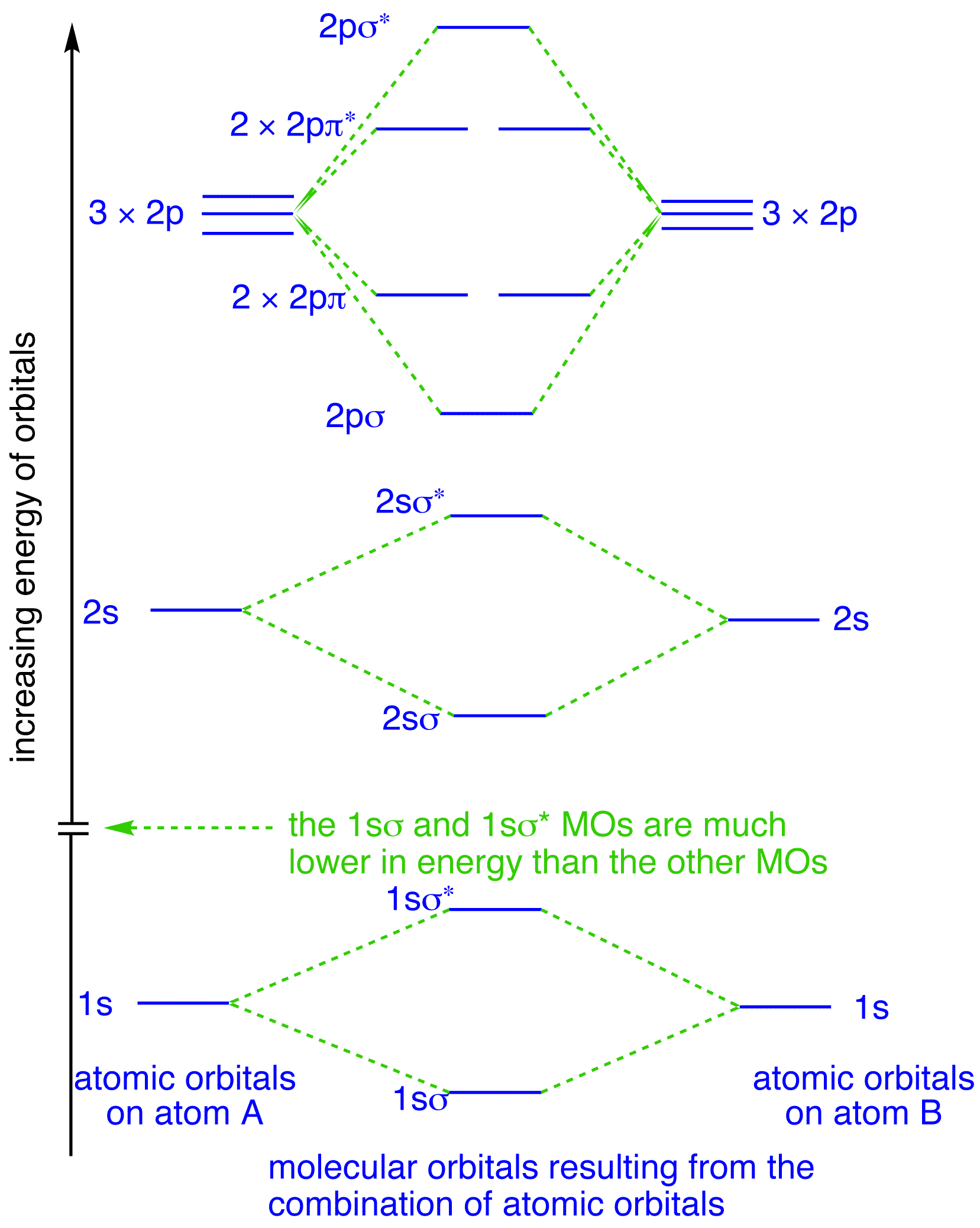
Methane molecular orbital diagram. Molecular Orbital Theory - Purdue University Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... (Get Answer) - Molecular orbital theory. Methane (CH4) has ... Molecular orbital theory. Methane (CH4) has tetrahedral geometry and Td point group symmetry. Derive a molecular orbital diagram for methane by performing the following steps: a. Determine the reducible representation (Γ) describing the symmetry of the four H 1s orbitals that are involved in σ bonding with the valence atomic orbitals of C. b. Ch4 Molecular Orbital Diagram - Wiring Diagrams Generate the Molecular Orbitals for CH4 (Td), CH4 (D4h) and Cyclopropane using diagram between the bonding MOs of square planar and tetrahedral CH4. The molecular orbital description of bonding in methane does several things for us. Here is an energy level diagram showing how the 4 hydrogen 1s orbitals.Jan 18, · Using LCAO to Construct MOs for ... Polyatomic Species: Molecular Orbitals - meta-synthesis Methane's MOs have a topology similar to the AOs of carbon, but the structure can be very difficult to visualise, so the methane MO construction diagrams A, B and C (below) are shown with the AOs and MOs superimposed upon line structures of the methane.
PDF Molecular Orbital Theory - Octahedral, Tetrahedral or ... molecular orbitals. 2.The number of molecular orbitals formed is the same as that of the number of atomic orbitals combined. 3.The additive overlap results in the bonding molecular orbital while the subtractive overlap results in the antibonding overlap. 4.The energy of bonding molecular orbitals is lower than their nonbonding counterparts ... Molecular Orbital theory: Ethane Ethane. • As molecules get bigger constructing the molecular orbitals. becomes more challenging. • Insights into bonding of larger molecules can be attained by. combining fragments with well defined MO's... through orbital. mixing. • In this manner, ethane can be constructed from MO's of two. pyramidal CH3 groups. Polyatomic Molecular Orbital Theory - La Salle University MO diagram of homonuclear diatomic molecules ... 4) MO theory and molecular geometry (Walsh diagrams) ... Molecular Orbital Theory – LGOs for methane.29 pages what type of hybrid orbital exists in the methane molecule ... What is the orbital structure of methane? Methane molecule consists of one carbon and four hydrogen atoms (CH4). Nature of Hybridization: In methane C-atom is Sp3-hybridized. One s-orbital and three p-orbitals (2px,2py,2pz) of carbon atom undergo Sp3-hybridization to produce four Sp3-hybrid orbitals.
Molecular Orbital Diagram Ch4 - schematron.org A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in . A Molecular Orbital Approach to Bonding in Methane methane (CH4) molecule . A molecular orbital diagram showing both the bonding and anti-bonding. Molecular Orbital Diagram (MO Diagram) of methane (CH4 ... Methane is a pentatomic, tetrahedral molecule. It is formed by combination of one carbon atom with 4 hydrogen atoms. In the molecule of methane, the carbon a... draw an orbital diagram of methane molecule - Chemistry ... Please explain me the orbital diagram and electron Dot structure of ccl4, h2o, nh3,ch4. why does graphite have high melting or boiling point if their bonding is weak. give one property of hydrogen chloride which agrees with it being a covalent compound. A covalent hydrocarbon molecule having four single covalent bond. Introduction to Molecular Orbital Theory Methane has four valence molecular orbitals (bonding), consisting of one orbital with one nodal plane (lowest occupied) and three degenerate (equal energy) orbitals that do have a nodal plane. For the energy diagram and pictorial view of the orbitals - please see below: Ethane:
Molecular orbitals - Single bonds, Methane The resulting angle between orbitals is 109.5°. Four Hydrogen atoms bond with Carbon to give methane. Hydrogen's spherical 1s orbital merges with one of Carbon's sp 3 orbitals to form a new molecular bonding orbital with Hydrogen's nucleus embedded in it. The bond produced from the overlap of the two atomic orbitals is called a sigma ...
Bonding in Methane - sp3 hybridisation | ChemKey The hydrogens bond with the two carbons to produce molecular orbitals just as they did with methane. The two carbon atoms bond by merging their remaining sp 3 hybrid orbitals end-to-end to make a new molecular orbital. The bond formed by this end-to-end overlap is called a sigma bond. The bonds between the carbons and hydrogens are also sigma ...
Supplementary Illustrations - Michigan State University Molecular Orbital Diagram for Methane Note that the carbon 1s orbital is omitted from the diagram, since it does not contribute to the bonding. Methane Molecular Orbitals In the following model, the carbon atom is dark gray and the hydrogens are cyan. The hydrogen atoms are arbitrarily numbered.
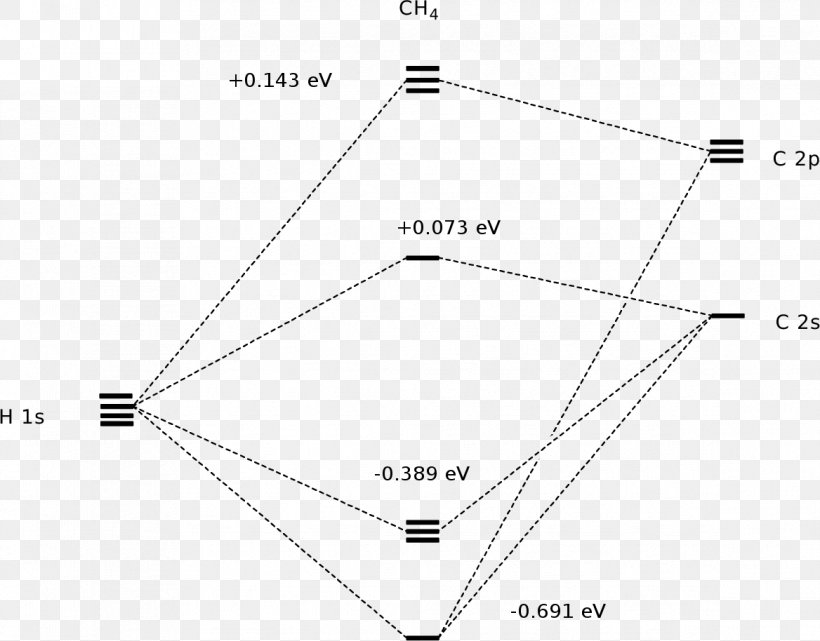
Methane Molecular orbitals | Methane (CH4) molecular ... Methane (CH4) molecular orbital diagram (sigma bonding and antibonding orbitals shown). Firefly 8.2.0 DFT B3LYP 6-311G(d,p) energy minimum (E = -40.5343 Hartrees), Td point group symmetry. MO 2 = -18.94 (1a1); 3-5 = -10.75 (1t2); 6 = 1.41 (2a1) and 7-9 = 3.38 eV (2t2). MOs 3-5 and 7-9 are degenerate, and for the bonding orbitals, valence electron density (2 electrons in each) is spread over ...
79 - Molecular orbitals methane - YouTube This animation explains the methane molecule through the Molecular Orbitals TheoryFor more Chemistry animations check out the youtube channel Lili Tosta: htt...
Ch4 Molecular Orbital Diagram - schematron.org A Molecular Orbital Approach to Bonding in Methane methane (CH4) molecule . A molecular orbital diagram showing both the bonding and anti-bonding. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals.
PDF A Molecular Orbital Approach to Bonding in Methane Methane has eight valence electrons, so according to the aufbau and Pauli exclusion principles the two lowest energy molecular orbitals (2a1 and 1t2) are fully occupied with electrons. This is consistent with the experimental valence electron ionization spectrum for methane shown below.
PDF Hybrid Molecular Orbitals - University of Illinois Urbana ... molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs. It is a linear molecule. Chemistry 104 ...
Molecular Orbitals of Square-Planar Tetrahydrides | VIPEr This in-class activity walks students through the preparation of a molecular-orbital diagram for methane in a square-planar environment. The students generate ligand-group orbitals (LGOs) for the set of 4 H(1s) orbitals and then interact these with carbon, ultimately finding that such a geometry is strongly disfavored because it does not maximize H/C bonding and leaves a lone pair on C.
Bonding orbitals in Methane - sp3 hybrids - ChemTube3D p-orbitals; 3p-orbitals; 3d-orbitals; 4f-orbitals; Compare shape and size of 1s, 2s and 2p orbitals; Molecular Orbitals. Hydrogen; Nitrogen; Fluorine; Ammonia; Methane; Ethylene (Ethene) Acetylene (Ethyne) Allene; Formaldehyde(Methanal) Acrolein; Carbon Monoxide; Hydrogen Fluoride; Allyl Anion; Butadiene; Benzene; Aromaticity of cyclic polyenes ...
3-MO theory(U).pptx Molecular Orbital Theory: Electrons are located in the molecule, ... Valence bond theory predicts four identical C-H bonds in methane.30 pages
Molecular Orbitals: Ethene (Ethylene) Molecular Orbitals: Ethene (Ethylene) Ethylene MOs. Ethylene is the simplest molecule that has a double bond. As we saw from the valence bond model, we should find the presence of a σ-bond framework, and a π-bond between carbons. Jmol._Canvas2D (Jmol) "Ethylene" [x]
PDF ORBITALS and MOLECULAR REPRESENTATION Construct the molecular orbital diagram for dichlorine. x y z z y 3 x y z z y 4 Showing the p orbitals. Showing the s and p orbitals. ORBITALS AND MOLECULAR REPRESENTATION 11. CARBON ORBITALS Methane Ethane METHANE AND ETHANE C H H H H CH4 C C H H H H H H C2H6 1 2 Color conventions: Hydrogen atoms are shown in gray.
PDF Deriving the MO diagram for square planar methane. square planar methane. With no lone pairs, the electronic and molecular geometry are the same. Technique for constructing LGO's: I Draw Lewis structure and assign VSEPR geometry (already done above) II Assign a point group to the molecular geometry III determine the central atom's VB hybrid orbitals for the electronic geometry
PDF Consider ethylene (also called ethene): C H . Draw a Lewis ... • Example - In methane, CH 4, the sp3 hybrid orbitals point at the 1s hydrogen orbitals, and the atomic orbitals are added and subtracted to create molecular orbitals • The other resulting MO is higher in energy than the two atomic orbitals and is antibonding • Only the lower-energy orbital is populated with electrons in methane


















0 Response to "38 methane molecular orbital diagram"
Post a Comment