39 lewis dot diagram for ccl4
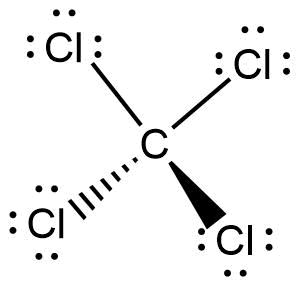
Ccl4 Lewis Structure How To Draw The Dot Structure For ... Carbon tetrachloride (ccl 4) is a covalently bonded compound composed of a central carbon surrounded by 4 chlorine atoms in a tetrahedral structure. the lewis diagram from carbon tetrachloride is: a regular atom of carbon has 4 lone electrons in its outer shell. chlorine has 7 electrons and so is 1 electron short of completely filling its outer. What is the Lewis dot structure for CCl4? - Answers The Lewis dot structure for CCl4 starts with a C in the middle. atom. On the unconnected sides of the Cl atoms, there are two dots, six in total on each atom. What does the Lewis dot structure for...
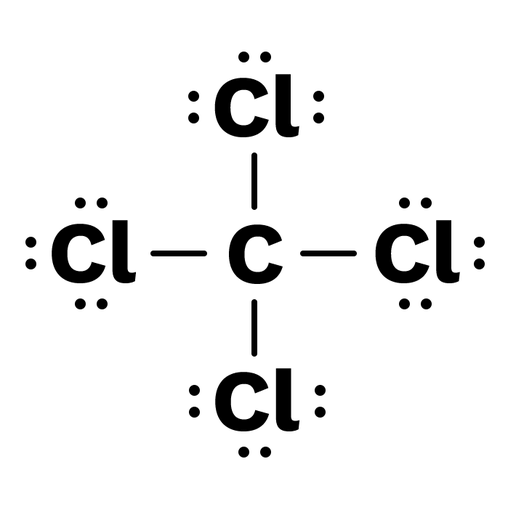
Carbon tetrachloride (CCl4) lewis dot structure ... - Topblogtenz CCl4 lewis’s structure is made up of one carbon atom that is situated at the middle position and four chlorine atoms that are at the surrounding position. The total lone pair present in the CCl4 lewis dot structure is 12. Lone pairs of electrons do not involve in chemical bonds and it is represented as a dot in the lewis diagram.
Lewis dot diagram for ccl4
Carbon tetrabromide (CBr4) lewis dot structure, molecular ... The lewis structure of CBr4 is similar to CCl4 and CF4, since, they all are in the same group in the periodic table and contains the same number of valence electrons. Follow some steps for drawing the lewis dot structure of CBr4. 1. Count total valence electron in CBr4. (PDF) Inorganic Chemistry by Miessler ~ 5th Edition ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. The necessary steps required to show the formation of CCl4 ... Click here👆to get an answer to your question ️ The necessary steps required to show the formation of CCl4 by Lewis electron dot diagram has been jumbled. Arrange them in a sequence.(a) Thus, an electron pair is shared between C and Cl . This is the Lewis electron dot diagram for CCl4 .(b) Write the symbol of chlorine and represent its valence electrons with the help of crosses, that is,(c ...
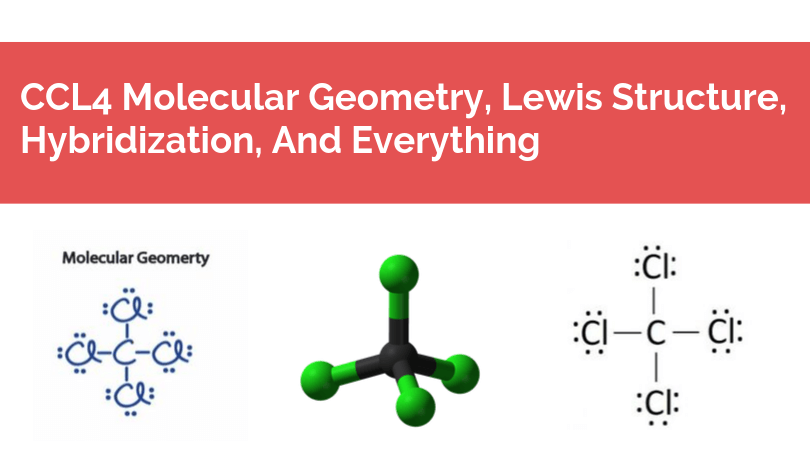
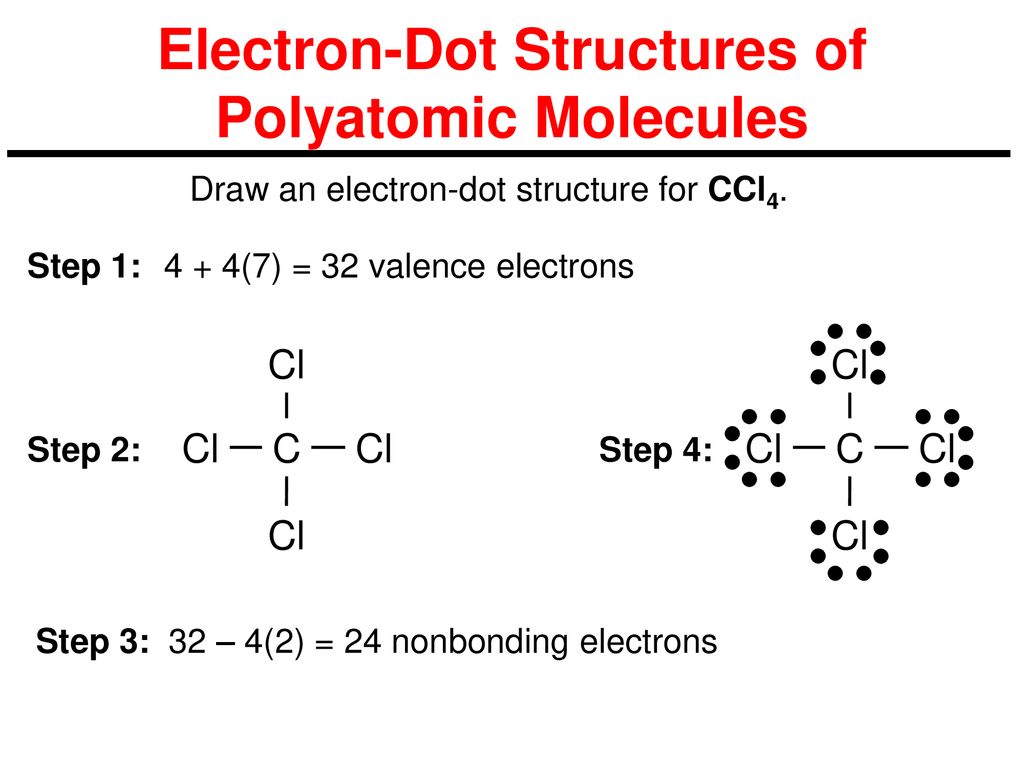
Lewis dot diagram for ccl4. CCL4 Molecular Geometry, Lewis Structure, Hybridization ... For the Lewis structure of CCl4 first, let's calculate the total valence electrons. Carbon has four valence electrons and each Chlorine atom has seven valence electrons. As there are four molecules of Chlorine, we will calculate the number of valence electrons accordingly. = 4 + (4*7) = 4 + 28 = 32 valence electrons What Is The Lewis Dot Diagram For CCL4? | Experts123 I will provide a link to a page that does illustrate the lewis structure for what is commonly referred to as cargon-tet. The following is a link to a site which provides a tutorial for writing lewis structures for a number of common molecules including the molecule requested as Carbon tetra-Cloride. Ch4 Dot Structure - lewis dot structure of ch4 methane ... Ch4 Dot Structure. Here are a number of highest rated Ch4 Dot Structure pictures upon internet. We identified it from obedient source. Its submitted by government in the best field. We allow this kind of Ch4 Dot Structure graphic could possibly be the most trending topic past we ration it in google help or facebook. toowhite.it Worksheet electron dot diagrams and lewis structures
PDF Lewis Dot Diagram Ccl4 - moviq.nl Lewis Dot Structure of CCl4 Carbon TetraChloride YouTube. CCl4 Lewis Structure How to Draw the Dot Structure for. Gacl4 lewis structure jtq atservice srl it. Which Diagram Best Represents A Polar Molecule Ekerekizul. How many lone pairs of electrons are in CCl4 eNotes com. Lewis Dot Structure and Polarity of CCl4 Carbon. Ccl4 lewis CCl4 Lewis Structure, Molecular Geometry, Hybridization ... 21/02/2022 · The Lewis structures are also known as Lewis dot diagrams or electron dot diagrams. This nomenclature is there because of the bond representation that takes place within the compound. Lone electrons are represented as dots in the Lewis structure, whereas, bonds are represented as a single line in the structure. All the electrons that take part in forming … Cl2 lewis structure, Molecular shape, Polar or Non-Polar ... Lewis dot structure for Cl2 (Chlorine gas) By looking at the above Cl2 lewis structure, we see both chlorine atoms completed their octet comfortably as both of them have 8 electrons around them. And no need to make any covalent bond in this lewis diagram because we got our stable lewis dot structure for Cl2. How to draw a CCl4 Lewis Structure? - Science Education ... In a CCl4 Lewis Structure diagram, the carbon atom can be the centre atom. As a result, central carbon in the CCl4 Lewis Structure, with all four Chlorines arranged around the tetrahedral geometry. Step-3: Combining step1 and step2 to get step3 for CCl4 dot structure
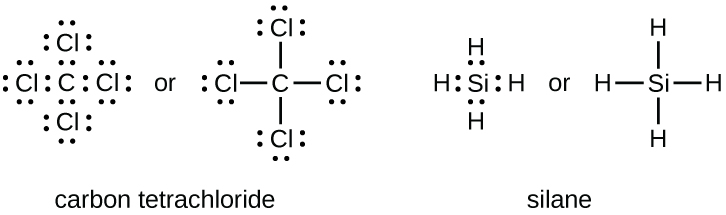
CCl4 Lewis Structure - How to Draw the Dot Structure for ... Drawing the Lewis Structure for CCl 4 (Carbon Tetachloride) Viewing Notes: The Lewis structure for CCl 4 is similar to CF 4. Since they are in the same Group on the periodic table they each have the same number of electrons (7) their structures are similar. For the CCl 4 Lewis structure there are a total of 32 valence electrons available. Solved Draw the Lewis structure for CCl4. What is the ... See the answer See the answer done loading. Draw the Lewis structure for CCl4. What is the molecular geometry of this compound? Is the molecule polar or nonpolar? Best Answer. This is the best answer based on feedback and ratings. 100% (7 ratings) tetra …. View the full answer. CCl4 Lewis Structure - Science Trends Nov 12, 2018 · A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound. Carbon tetrachloride (CCl 4) is a covalently bonded compound composed of a central carbon ... PowerPoint Presentation - Chemical BONDING PowerPoint Presentation Draw Lewis Dot Structures Draw the Lewis Dot Diagram for polyatomic ions Draw Polyatomics Types of Covalent Bonds Types of Covalent Bonds Place these molecules in order of increasing bond polarity which is least and which is most? non-polar MOLECULES Polar molecules (a.k.a. Dipoles) PowerPoint Presentation Space filling model …
Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3.
SiO2 Lewis Structure, Molecular Geometry, Hybridization ... 20/02/2022 · This is the last step in completing the Lewis diagram for SiO2. As we all know, silicon needs 8 electrons to complete its octet, but it only has 4 electrons right now. By turning an oxygen atom’s electrons into a covalent bond, we would be able to achieve the octet of silicon. We convert two lone pairs of electrons from each oxygen atom to a covalent bond, as seen in …
Lewis Dot of Carbon TetraChloride CCl4 TetraChloromethane Because of this symmetrical geometry, CCl4 is non-polar. Methane gas has the same structure, making carbon tetrachloride a halomethane. As a solvent, it is well suited to dissolving other non-polar compounds, fats and oils. It can also dissolve iodine. It is somewhat volatile, giving off vapors having a smell characteristic of other chlorinated ...
DOC Hudson City School District Step 1: Build a Lewis dot structure for ozone, O3 Total valence electrons (3(6) = 18 Steps 2 and 3: Place one O in the center, and connect the other two O's to it. Drawing a single bond from the central atom to each of the surrounding atoms.
What is the molecular geometry of CCl4? Draw its VSEPR and ... The Lewis structure shows that there are four electron regions about the central carbon atom. The VSEPR model states that the electron regions around an atom spread out to make each region is as far from the others as possible. For these clouds in CCl4 to be as far as possible from one another, they will point toward the corners of a tetrahedron.
Lewis Dot Structure and Polarity of CCl4 (Carbon Tetrachloride) Lewis Dot Structure for CCl4 The Lewis dot structure diagram depicts the placement of electrons in the molecules of any compound. The electrons are represented with the help of circular dots. This diagram displays the bonds formed as well as lone pairs of electrons. Consider the diagram given above.
Silicon dioxide (SiO2) lewis dot structure ... - Topblogtenz Silicon dioxide (SiO2) lewis structure. As you see in the above SiO2 lewis dot structure, we convert 2 lone pairs of electrons of each oxygen atom to a covalent bond. So, both atoms (silicon and oxygen) have 8 electrons in their valence shell. Hence we got our best and stable Silicon dioxide lewis structure.
Lewis structure for CCl4? - Answers The Lewis dot structure for CCl4 starts with a C in the middle. atom. On the unconnected sides of the Cl atoms, there are two dots, six in total on each atom. Home Study Guides Science Math and...
Lewis Structure Questions and Answers | Study.com Lewis Structure Questions and Answers. Get help with your Lewis structure homework. Access the answers to hundreds of Lewis structure questions that are explained in a …
CCl4 Lewis Structure - How to Draw the Dot Structure for CCl4 ... A step-by-step explanation of how to draw the CCl4 Lewis Dot Structure (Carbon tetrachloride). The Lewis structure for CCl4 is a commonly tested Lewis struc...
Draw the Lewis dot structures of N2 and CCl4 - Brainly.in 2) Lewis dot structure of CCl4 is given below: It has a tetrahedral geometry. first we have to draw the skeleton structure in which the other atoms are single-bonded to the central atom — a C atom with four Cl atoms attached to it. And then draw a trial structure by putting electron pairs around every atom until each gets an octet. Advertisement
shoppyapp.it Il y a 2 jours · A Lewis structure (or electron-dot formula) is a two-dimensional structural formula showing the arrangement of electrons around atoms in covalently bonded molecules—i. Between Tigo (Millicom Ghana Limited) & Databank, Barnes road, Ridge. National Library of Medicine. Permalink. ODraw the Lewis structure of each molecule and count the number of nonbonding …
Lewis Dot Structure of CCl4 (Carbon TetraChloride) - YouTube I quickly take you through how to draw the Lewis Structure of CCl4 (Carbon TetraChloride). I also go over hybridization, shape and bond angle.
omerosecchiari.it Il y a 2 jours · CHEM 1A: VSEPR Theory Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and polyatomic ions, we will use Lewis dot structures to predict electronic and molecular geometries. In H 2 O, only two of the six outer-shell electrons of oxygen are used for this purpose, leaving four electrons which are organized into …
What is the Lewis dot structure for CCl4? - Studyrankersonline 0 votes. 975 views. asked Dec 11, 2019 in Important Questions by megha00 Expert (17.8k points) What is the Lewis dot structure for CCl4?
PDF Lewis diagram for ccl4 - varida-tech.com Lewis Dot Structure and polarity of CCl4 (Carbon Tetrachloride) Carbon tetrachloride, also known as tetrachloromethane, is a compound containing carbon and chlorine. A structure of Lewis CCL4 is a diagram that represents the configuration of electrons of compounds covalently bound. Lewis's structures are intended to provide a
Draw the Lewis structure for CCl4.... | Clutch Prep We're being asked to draw a Lewis structure for CCl 4.. To do so, we first need to do the following steps: Step 1: Determine the central atom in this molecule. Step 2: Calculate the total number of valence electrons present. Step 3: Draw the Lewis structure for the molecule.
CCl4 Lewis Structure | Science Trends - World Tribune CCl4 Lewis Structure | Science Trends May 14, 2021 Posted by artnews No Comments. Lewis dot structures help predict molecular geometry. This example problem shows the steps to draw a structure where an atom violates the octet rule. "Lewis Structures and the Octet Rule. ... How to draw the Lewis Dot Structure for NO+.
The necessary steps required to show the formation of CCl4 ... Click here👆to get an answer to your question ️ The necessary steps required to show the formation of CCl4 by Lewis electron dot diagram has been jumbled. Arrange them in a sequence.(a) Thus, an electron pair is shared between C and Cl . This is the Lewis electron dot diagram for CCl4 .(b) Write the symbol of chlorine and represent its valence electrons with the help of crosses, that is,(c ...
(PDF) Inorganic Chemistry by Miessler ~ 5th Edition ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry.
Carbon tetrabromide (CBr4) lewis dot structure, molecular ... The lewis structure of CBr4 is similar to CCl4 and CF4, since, they all are in the same group in the periodic table and contains the same number of valence electrons. Follow some steps for drawing the lewis dot structure of CBr4. 1. Count total valence electron in CBr4.



















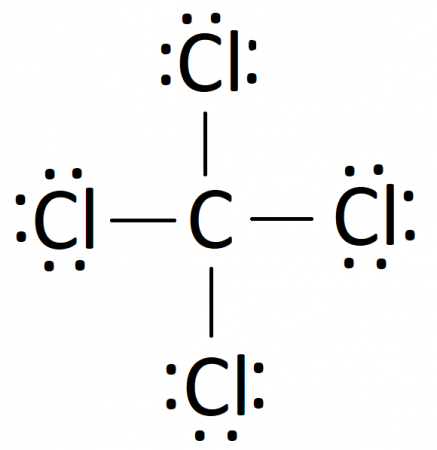








0 Response to "39 lewis dot diagram for ccl4"
Post a Comment