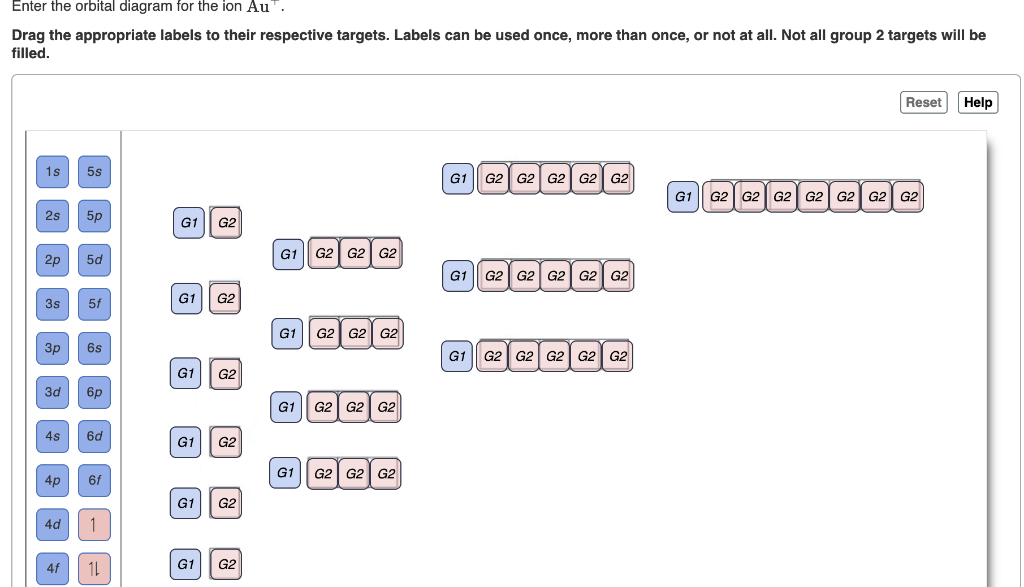
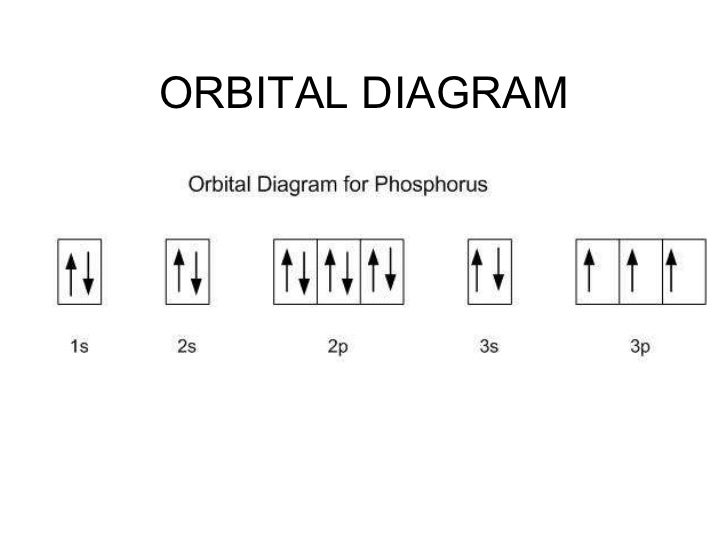
40 enter the orbital diagram for the ion au+.
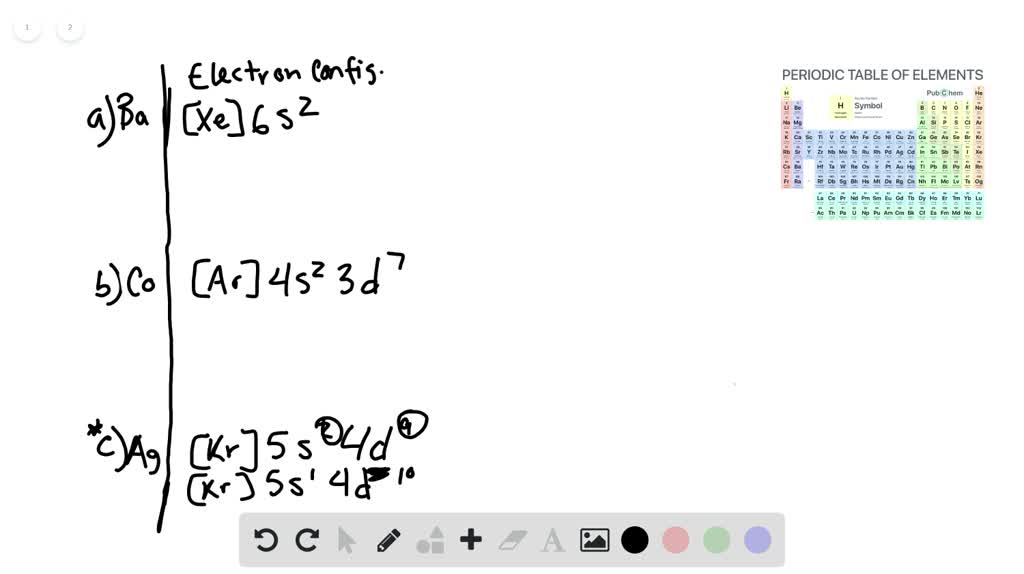
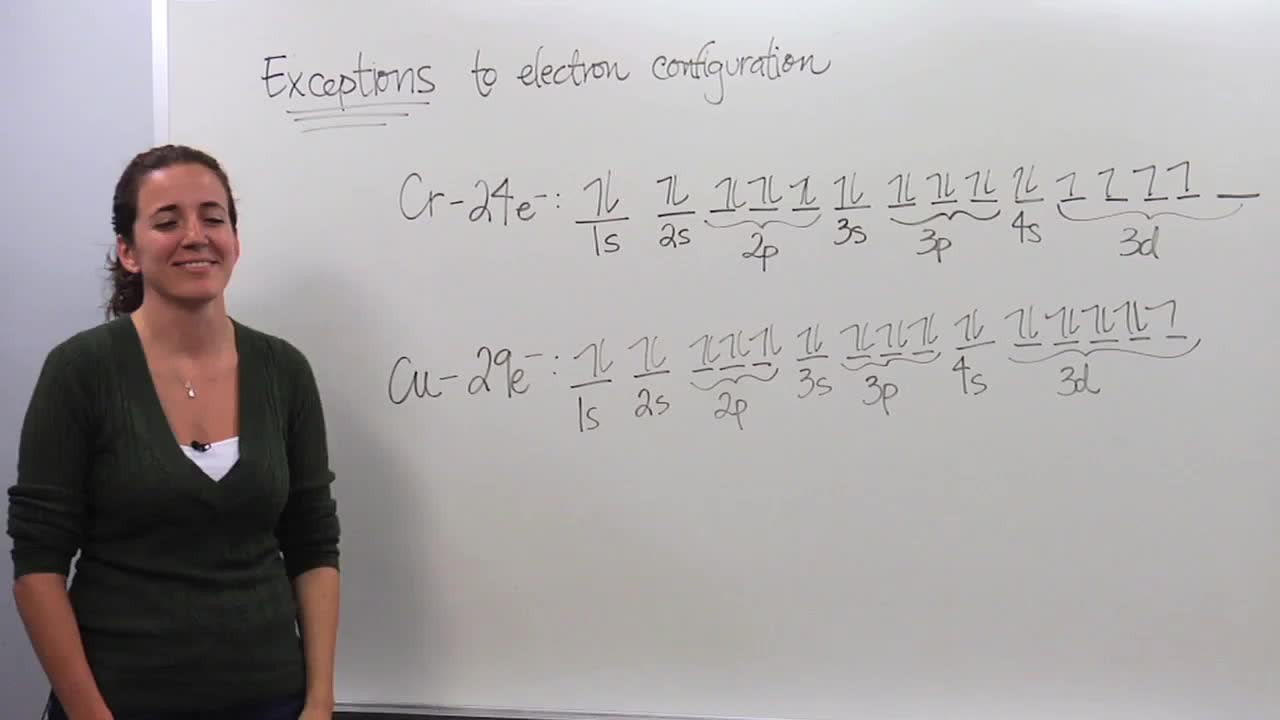
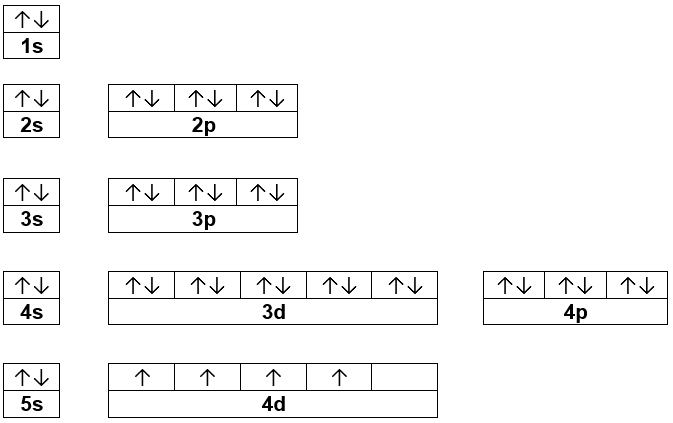
Best answer. orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. you just have to fill the "boxes" with arrows, s orbital has only one box with two arrows; p has 3orbitals with 6arrows; d has 5 boxes with 10 arrows and f has 7boxes with 14 arrows. In each box can be two arrows with opposite spin maximum! Enter the orbital diagram for the ion Cd2+Cd2+. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be filled. Enter the orbital diagram for the ion Au+Au+. Drag the appropriate labels to their respective targets.
The atomic number of Au is Therefore, its For Au+, one electron is removed from the outermost 6s orbital, making the configuration. Electron Configuration, [Xe] 4f14 5d10 6s1. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. Orbital Diagram. 1s. ↿⇂. 2s. ↿⇂. 2p. Write orbital diagram for Au+? ↿⇂. ↿⇂. ↿⇂.

Enter the orbital diagram for the ion au+.
What atomic or hybrid orbitals make up the sigma bond between As and F in hexafluoroarsenate ion, AsF6- ? orbital on AS:? orbital on F:? Chemistry. How can the ...2 answers · Top answer: [Xe] 4f14 5d10 Always follow this pattern: 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s... Part 1. Hence, the orbital diagram for ion is as follows: Part 2. Therefore, cation is diamagnetic in nature. Ground state electron configuration for Au: 7. Au : 1s%2s²2p3s²3pº3d" 4s²4p 4d' 5s-5p4f"50"6s' Excited state electron configuration for Au* ion : ,Aut : 1s²2s²2p%3s 3p%3d" 4s24pº4d"°58²5p®48'45d". Question: Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. This problem has been solved! See the answer ...
Enter the orbital diagram for the ion au+.. the atomic number of au is therefore, its for au+, one electron is removed from the outermost 6s orbital, making the configuration. orbital diagram for au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the "boxes" with arrows, s orbital.orbital diagrams of atoms diagram shows how the electrons are distributed … Science. Chemistry. Chemistry questions and answers. Enter the orbital diagram for the ion Cd2+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be filled. Enter the orbital diagram for the ion Au+. Drag the appropriate labels to their respective targets. What is the orbital diagram for Au? ↿⇂. ↿⇂. Answer to Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. Similar Questions. Chemistry. (1) Which of the following clusters of orbitals would form the shape trigonal bipyramidal and would also be possible within the.May 06, · Write orbital diagram for Au+? ... You are watching: Enter the orbital diagram for the ion au+. This is a memory device to remember the order of orbitals for the first two quantum numbers. Follow the arrow starting in the upper right, when the arrow ends go to the next arrow and start again.
The atomic number of Au is 79. Therefore, its configuration is: #1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^2 5p^6 4f^14 5d^10 6s^1# or, #[Xe] 4f^14 5d^10 6s^1# For #Au^+#, one electron is removed from the outermost #6s# orbital, making the configuration, #[Xe] 4f^14 5d^10# 1 answerThe give cation is Au+ A u + named as gold ion. The atomic number in periodic table is 79. Electronic configuration... Enter the orbital diagram for the ion Zr2+ ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed from the "s" sub-level 1.) Remove 2 electrons from 5s2 ANSWER: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d2. Identify whether the ions are diamagnetic or paramagnetic. Question: Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. This problem has been solved! See the answer ...
Part 1. Hence, the orbital diagram for ion is as follows: Part 2. Therefore, cation is diamagnetic in nature. Ground state electron configuration for Au: 7. Au : 1s%2s²2p3s²3pº3d" 4s²4p 4d' 5s-5p4f"50"6s' Excited state electron configuration for Au* ion : ,Aut : 1s²2s²2p%3s 3p%3d" 4s24pº4d"°58²5p®48'45d". What atomic or hybrid orbitals make up the sigma bond between As and F in hexafluoroarsenate ion, AsF6- ? orbital on AS:? orbital on F:? Chemistry. How can the ...2 answers · Top answer: [Xe] 4f14 5d10 Always follow this pattern: 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s...









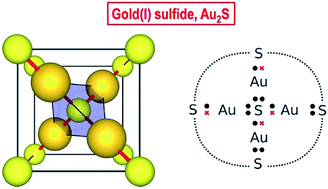








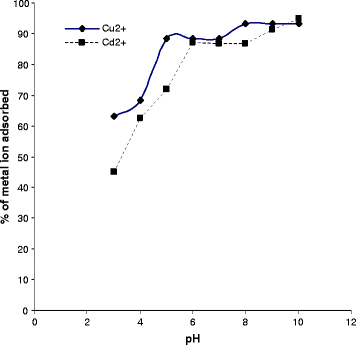








0 Response to "40 enter the orbital diagram for the ion au+."
Post a Comment