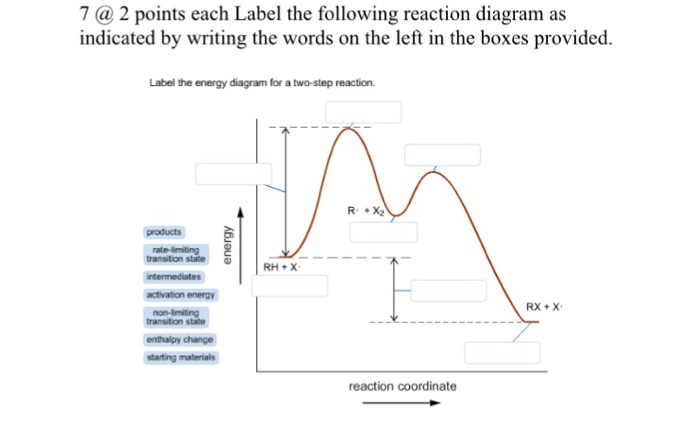
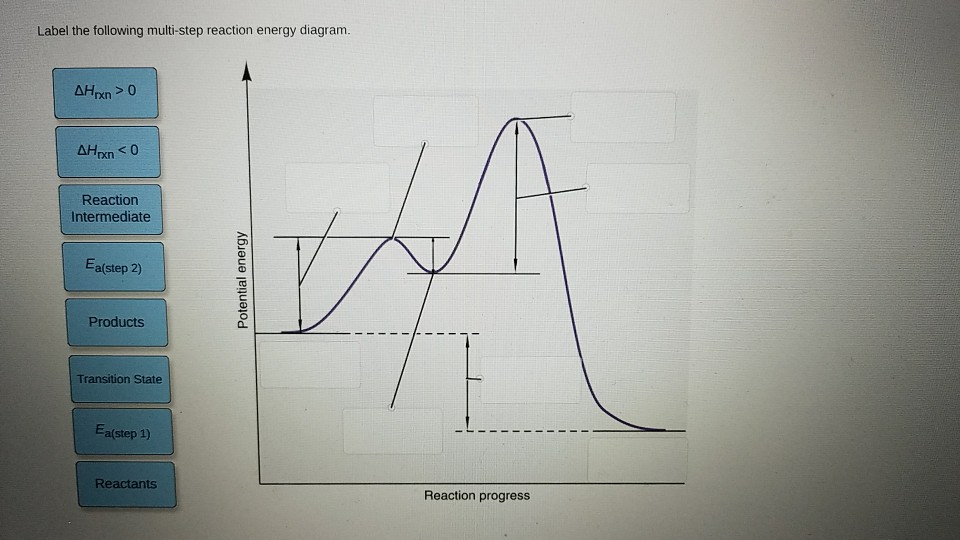
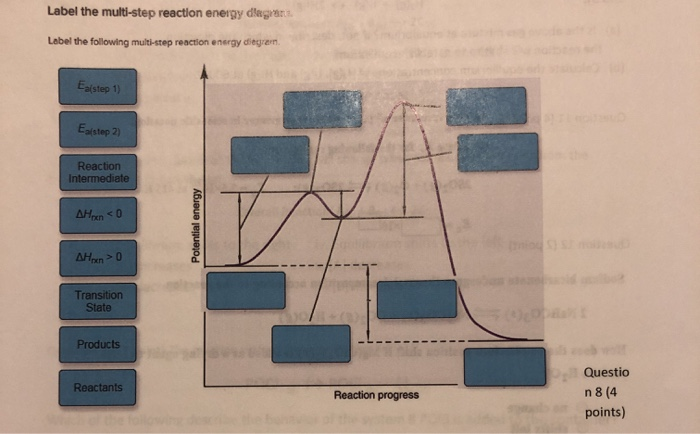
38 label the following multi-step reaction energy diagram.
3) For the following reaction coordinate (or energy diagram): ffÐ/uct5 energy a. b. d. ccadd/tltr Draw in x- and y-axes and label them. Label starting material$products,renergy change (AG), transition state, and activation energy (AGI.).vZ Is this reaction exergonic or endergonic? endergonlc produd< > eneyqyoérenctunrs Draw the MO diagram for the . valence electrons of N. 2. Label the (ii) atomic and (iii) molecular orbitals, including the x, y, and z designations where appropriate. (iii) Fill both the atomic and molecular orbitals with the proper number of electrons. (iv) Draw and label the Energy axis. Use the full space available to spread out your energy levels. (c)
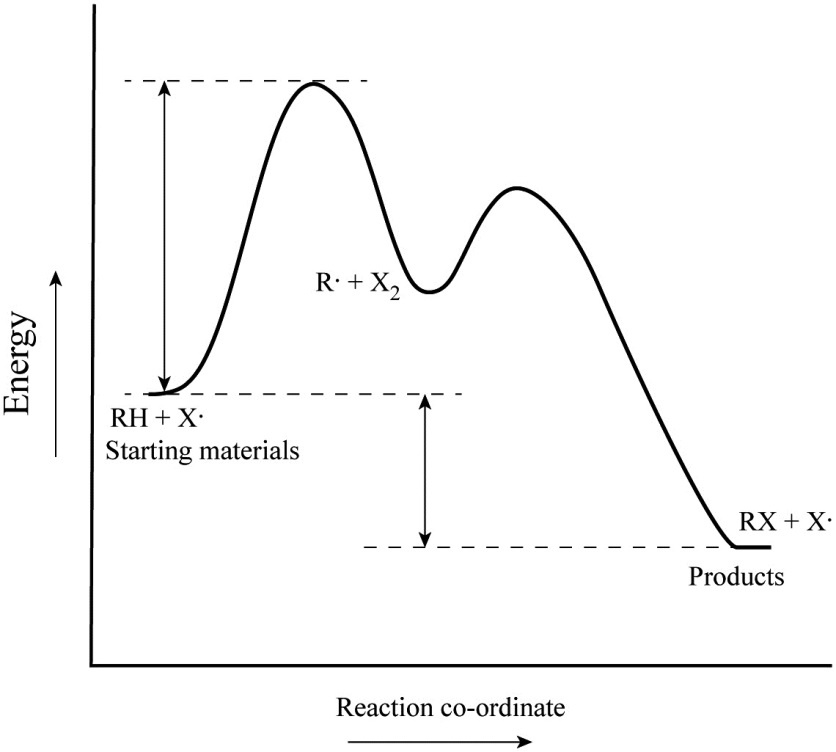
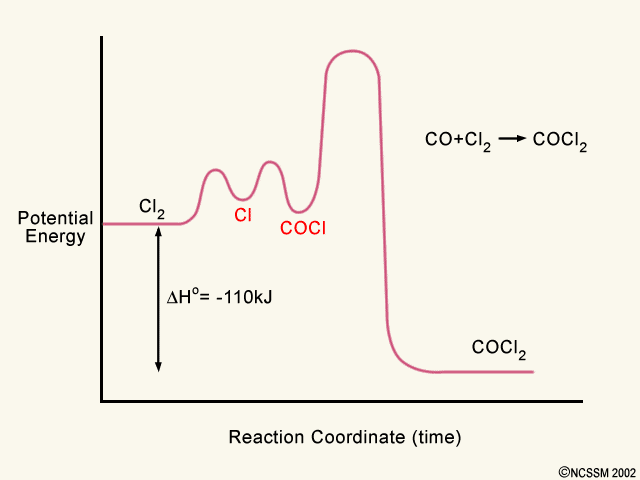
A possible reaction might happen in the following four steps, with X and Y existing temporarily unit they are used in a later step: (1) 2C X (fast) (2) B + X E (fast) (3) B + 2C D + Y (slow) (4) A + Y D (fast) Canceling the reaction intermediates and combing what is left, results into an equation which matches the overall balanced equation.

Label the following multi-step reaction energy diagram.
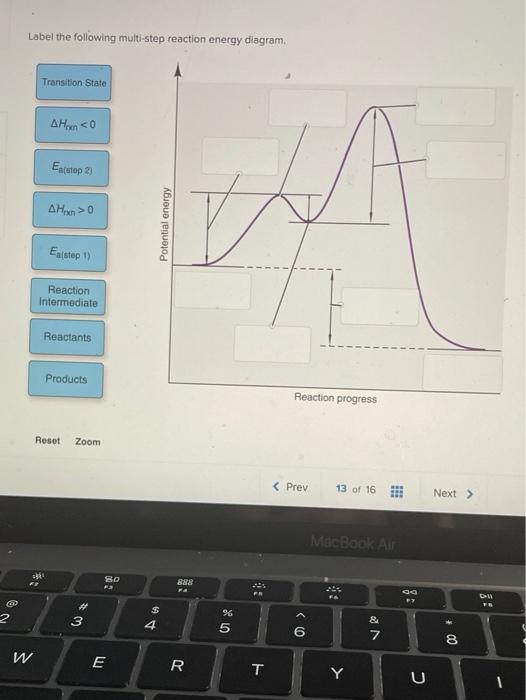
a. For each reaction: 1) Draw in all non-bonding electrons in the reactants and products. 2) Label each reactant as either nucleophile or electrophile. 3) Draw the curved arrows needed to show each reaction mechanism. b. Draw a free energy diagram for the reaction of ethanol with HBr to give bromoethane. Because a reaction cannot proceed faster than its slowest step, this step will limit the rate at which the overall reaction occurs. The slowest step is therefore called the rate-limiting step (or rate-determining step) of the reaction Figure 2. Figure 2. A cattle chute is a nonchemical example of a rate-determining step. Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (4 ratings) Transcribed image text: Label the following multi-step reaction energy diagram AHn> 0 Reaction Intermediate Ealstep 2) Products Ea (step 1) Reactants Reaction progress.
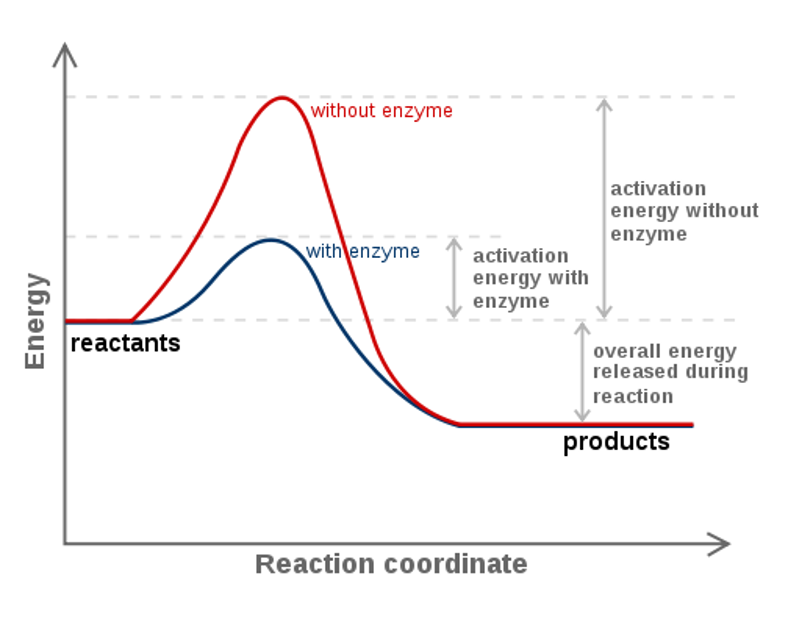
Label the following multi-step reaction energy diagram.. 2. Draw a potential energy (E p ) diagram for a reaction in which ∆H = 80 kJ/mol and E a = +28kJ/mol. Label the axes, activation energy, ∆H, site of the activated complex, reactants and products. Your starting value for the reactants might be different that is ok, as long as you Label the diagram with the appropriate terms to describe glycogen synthase regulation. ... The multi‑step activation of a fatty acid involves the hydrolysis of the equivalent of two molecules of ATP. The overall free energy change for the reaction includes the free energy change for the hydrolysis of ATP and the subsequent hydrolysis of PPi. Diagrams like this are described as energy profiles.In the diagram above, you can clearly see that you need an input of energy to get the reaction going. Once the activation energy barrier has been passed, you can also see that you get even more energy released, and so the reaction is overall exothermic. The second step is rate-determining. According to Wikipedia:. Given a reaction coordinate (energy diagram), the rate determining step can be determined by taking the largest energy difference between any starting material or intermediate on the diagram and any transition state that comes after it.
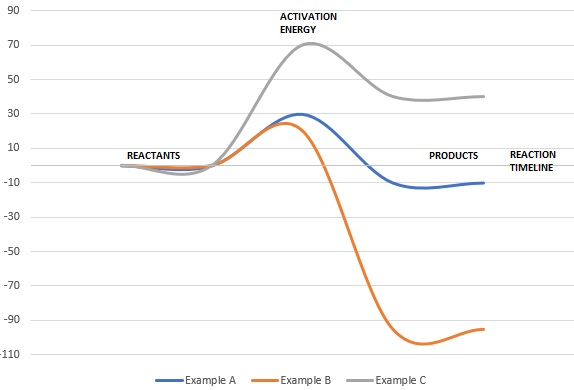
Cellular Respiration Equation: Every machine needs specific parts and fuel in order to function. Likewise, "biological machines" also require well engineered parts and good energy source in order to work.Perhaps the second most important molecule (DNA is the first) is adenosine triphosphate (also known as ATP).Basically, ATP serves as the main energy currency of the cell. This is part 3 of a four part series in the Energy Diagram Module. Stay tuned for Part 4! Click on the following links to see earlier parts: Part 1. Part 2. Sometimes reactions are more complex than simply a transition state (Graph 3), which would represent a single step in the reaction mechanism. Draw the potential energy diagram for the following multi-step reaction . Properly label the diagram. Solution: Rate of Reaction is Determined by Slowest Step In a series of reactions that make up a multi-step reaction, each individual reaction step has its own reaction rate that is determined by the factors that have been discussed in this chapter. Label the following multi-step reaction energy diagram. delta Hrxn > 0, Ea (step 2), Products, delta Hrxn < 0, Ea (step 1), Transition State, Reaction Intermediate, Reactants. Learn this topic by watching Energy Diagram Concept Videos.
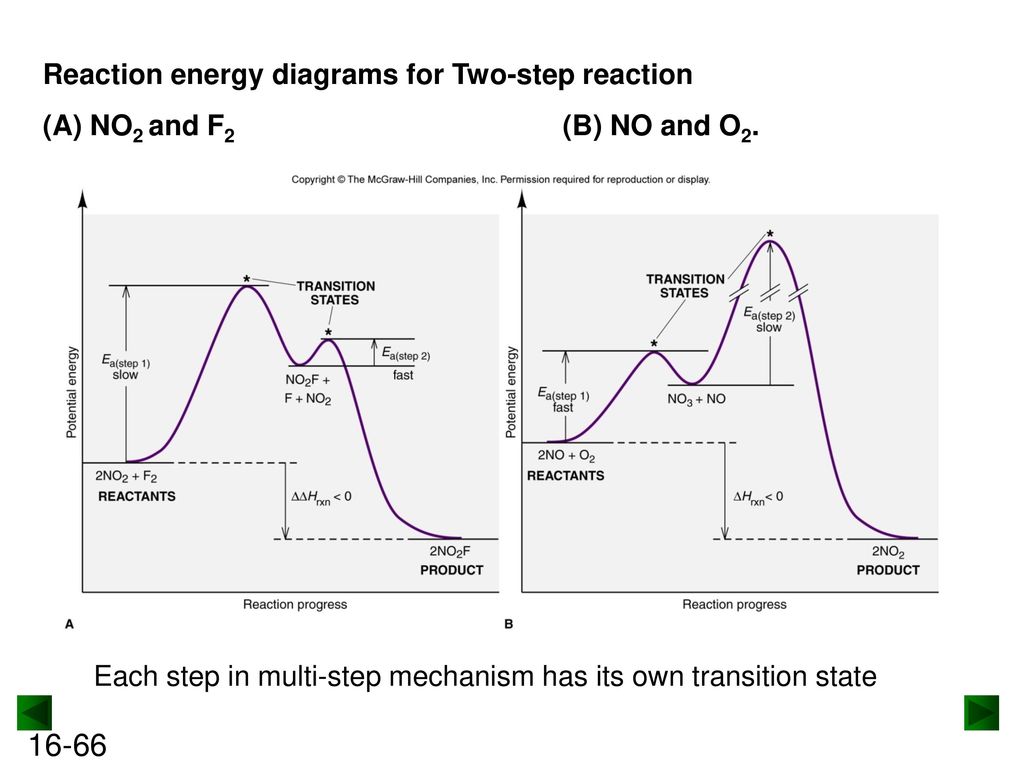
A Two-Step Reaction Mechanism. The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall. Step 1 has the higher transition energy state, thus it ... step in a multistep reaction. EXAMPLE 5.1 Draw an energy diagram for a two-step exothermic reaction in which the second step is rate determining. STRATEGY A two-step reaction involves the formation of an intermedi-ate. In order for the reaction to be exothermic, the products must be lower in energy than the reactants. In order for the Draw an energy diagram that represents the rearrangement of food in our bodies in Doodle Box A. Remember to label INPUTS (reactants=food) and OUTPUTS (products), and write what we know about them. www.modelbasedbiology.com The chemical reaction inside of us… 4 We know that we get energy from food through some kind of chemical reaction. Label the multi-step reaction energy diagram below using the letters corresponding to the labels on the left. There are more labels than needed; each label can be used only once. A) Reaction intermediate. B) E a (step 2) C) Reactants. D) Products. E) Transition state. F) E a (step 1) G) Catalyst. H) Equilibrium. I) k f. J) k r. K) Reaction coordinate. L) Energy
reaction. Use fishhook arrows! • Draw and completely label a reaction-energy diagram. • Deter min e th ra te-d ter ing sp of a ulti- ep re act ion-energy d gram. • Differentiate between transition states and intermediates. • Differentiate between kinetic and thermodynamic control. • Use the Hammond postulate to predict whether a
This potential energy diagram shows the effect of a catalyst on the activation energy. The catalyst provides a different reaction path with a lower activation energy. As shown, the catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two ...
• Accomplish catalysis without being consumed in the reaction. • Catalyzes a specific chemical reaction. The Enzyme in Action Kit© allows you to explore how enzymatic reactions occur. Catabolism Model pieces needed 1. The gray foam piece is a model of an enzyme. Place it with the A label facing up. Assemble the two green pieces (B 1 and B 2
2. Draw a potential energy (E p ) diagram for a reaction in which ∆H = 80 kJ/mol and E a = +28kJ/mol. Label the axes, activation energy, ∆H, site of the activated complex, reactants and products. 3. Using the potential energy diagrams for an endothermic and
Draw a free energy diagram for the acid-catalyzed hydration of 2-butene (the last step is very fast.) Label all reactants, products and intermediates using structures. 3. Reactions. a. Draw the major product or products of each of reaction in the boxes provided. ... Be explicit when showing multi-step reactions. 4. Mechanisms.
Label the following multi-step reaction diagram. Show transcribed image text. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 99% (110 ratings) Transcribed image text: Label the following multi-step reaction energy diagram.
Specifically, energy diagrams tell you about the relative rate of a reaction, or of any step in a reaction, and they tell you about thethermodynamic favorability of a reaction, or of any step in a reaction. Take a few minutes to review the four basic types of single-step processes that can occur, shown in Fig.2:
a. For each reaction: 1) Draw in all non-bonding electrons in the reactants and products. 2) Label each reactant as either nucleophile or electrophile. 3) Draw the curved arrows needed to show each reaction mechanism. b. Draw a free energy diagram for the reaction of ethanol with HBr to give bromoethane.
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (4 ratings) Transcribed image text: Label the following multi-step reaction energy diagram AHn> 0 Reaction Intermediate Ealstep 2) Products Ea (step 1) Reactants Reaction progress.
Because a reaction cannot proceed faster than its slowest step, this step will limit the rate at which the overall reaction occurs. The slowest step is therefore called the rate-limiting step (or rate-determining step) of the reaction Figure 2. Figure 2. A cattle chute is a nonchemical example of a rate-determining step.
a. For each reaction: 1) Draw in all non-bonding electrons in the reactants and products. 2) Label each reactant as either nucleophile or electrophile. 3) Draw the curved arrows needed to show each reaction mechanism. b. Draw a free energy diagram for the reaction of ethanol with HBr to give bromoethane.



















0 Response to "38 label the following multi-step reaction energy diagram."
Post a Comment