37 molecular orbital diagram practice problems with answers
(c) Molecular orbitals are generally described as being more delocalized than hybridized atomic orbitals. (d) One of the shortcomings of molecular orbital theory is its inability to account for a triple bond in the nitrogen molecule, N 2. (e) One of the shortcomings of valence bond theory is its inability to account for the paramagnetism of the ... Practice Problems D: Orbitals & molecules ... Use a qualitative molecular orbital energy-level diagram to describe the bonding in ... Explain your answer.4 pages
[Book 1 of The HEL Jumper](https://www.reddit.com/7oulr8) [Book 2 of The HEL Jumper](https://www.reddit.com/akws1r) \----- [Previous](https://redd.it/idztze) | [First](https://www.reddit.com/eo9svn) | [Next](https://redd.it/iqrcc1) | [Patreon](https://www.patreon.com/SabatonBabylon) Thanks to Big_Papa_Dakky, Darth_Android, bloblob, AMERICUH, The_Real_Jumper, Mr_Polygon, Krystalin, Damned_Thrice, Mamish, Vikairious, Sam_Berry, RedHawkdude, KillTech, LilLaussa, Daddy_Talon, Gruecifer, Gaelan_D...

Molecular orbital diagram practice problems with answers
Chapter 11 Answers Practice Examples 1a. There are three half-filled 2p orbitals on N, and one half-filled 5p orbital on I. Each half-filled 2p orbital from N will overlap with one half-filled 5p orbital of an I. Thus, there will be three N—I bonds. The I atoms will be oriented in the same direction as the three 2p orbitals of N: toward the x ,y, and z-directions of a Cartesian coordinate ... All Organic Chemistry Practice Problems Molecular Orbitals Practice Problems Q. Draw a molecular orbital energy diagram when the 1s orbitals of two hydrogen atoms combine to form a hydrogen molecule.In your drawing, label the... Feb 15, 2015 · February 15, 2015. By Marsha Massey. University of Sydney has created a practice website for reviewing different parts of molecular orbital diagrams. Using this resource you can add pieces to pre-drawn MO diagrams for over 20 different molecules. The site includes opportunities to practice filling in electrons, attaching the names/symbols of ...
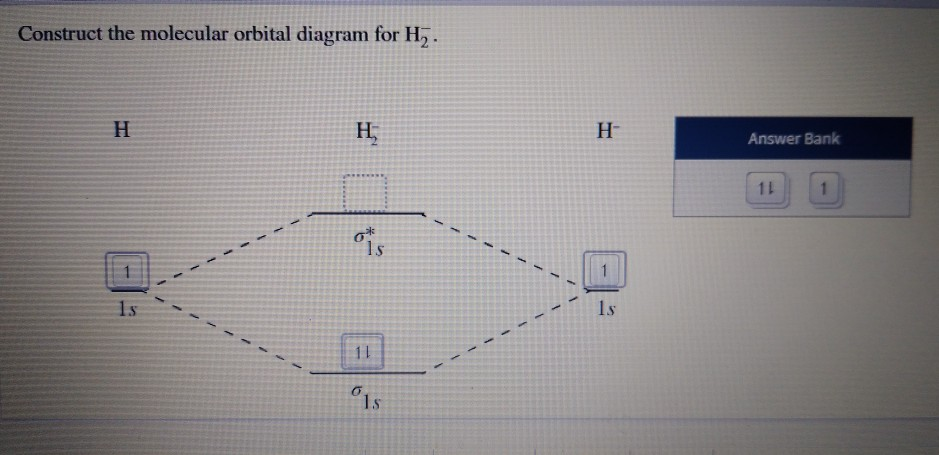
Molecular orbital diagram practice problems with answers. Chemistry 401 Intermediate Inorganic Chemistry University of Rhode Island Practice Problems Molecular Structure and Covalent Bonding. 1. Write Lewis structures for (a) XeF 4, (b) PF 5, (c) BrF 3, (d) TeCl 4, (e) ICl 2 –.Give the formal charge and oxidation number for each atom. These problems are for practice in drawing your molecular orbital diagrams, molecular electron configurations and determining bond order. The following questions pertain to the F2 molecule: A) Draw the molecular orbital energy diagram for this molecule. Label all of the orbitals specifically. *2px Answers to Practice Test Questions 3 . Molecular Orbital Theory: Heteronuclear Diatomic Molecules . 1. (a) 1The electron configuration for 𝐻𝐻 is 1𝑠𝑠, so 𝐻𝐻 has 1 valence electron. The electron configuration for 𝐻𝐻𝐻𝐻 is 1𝑠𝑠2, so 𝐻𝐻𝐻𝐻 has 2 valence electrons. (Circle your answer.) trigonal planar trigonal pyramidal (b) Draw both molecular orbital diagrams to explain your answer to part (a). You may ignore the core electrons and only show the orbitals where the valence electrons reside plus the LUMO (you do not need to show the higher, unoccupied molecular orbitals).
molecular orbital diagrams of diatomics worksheet in chemistry molecular orbital mo theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms but are treated as moving under the influence of the nuclei in the whole molecule in this theory each molecule has a set of molecular orbitals molecular orbital theory v practice with sigma and pi mo practice with the molecular orbitals of molecules by linear binations of 2s and 2p ... configurations and determining bond order. The following questions pertain to the F2 molecule: A) Draw the molecular orbital energy diagram for this molecule. › Molecular orbital theory practice problems › Molecular orbital diagram practice. About Molecular Orbital. In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability ... The molecular orbital diagram for ClO – is given below: The basis orbitals for Cl are 3s and 3p and for O are 2s and 2p. Z* for O 2s and 2p orbitals are similar so the AOs start at nearly the same energy. For the Cl 3s and 3p orbitals the two Z* values are quite different so the initial energies are more separated.
Recall that the 2p atomic orbitals on C and O may form molecular orbitals of both s and p symmetry. 9. The correlation diagram in Problem 7 correlates the separated atom orbitals for R = ¥ with the molecular orbitals at R e, the equilibrium internuclear distance in the molecule. Continue the correlation of the orbitals to the limiting case of ... Multiple choice questions by Catherine E. Housecroft. This activity ... In an MO diagram for the formation of H2O in which the z axis bisects the H-O-H angle: .... MCQ quiz on Chemistry multiple choice questions and answers on. Chemistry MCQ questions quiz on ... Theories | Molecular Orbital Valence Bond Model vs. Molecular Orbital Theory . Because arguments based on atomic orbitals focus on the bonds formed between valence electrons on an atom, they are often said to involve a valence-bond theory..The valence-bond model can't adequately explain the fact that some molecules contains two equivalent bonds with a bond order between that of a single bond and a double bond. molecular orbital diagram practice problems with answers questions amp answers 5 ask the physicist. resolve a doi name. heating and cooling curves ap chemistry. alkene reactivity department of chemistry. molecular orbitals in inorganic chemistry hunt research. what makes a good nucleophile master organic chemistry. chemistry 101science com.
4.3: Empirical and Molecular Formulas (Problems Jun 09, 2014 · Worksheet 6.2 The mole and chemical formulae Exercise 6.3 Calculating the percentage of certain elements in a compound and empirical formulae Worksheet 6.5 Empirical formulae and Worksheet 6.6 Molecular orbital diagram practice worksheet
8 Mar 2021 — Practice energy diagrams for molecular orbital theory. · Calculate the number of bonding and antibonding electrons in simple molecules.
Refer to the MO Diagrams. Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a hero nuclear diatomic molecule, ...9 pages
CHEM 2000 Exercises and Practice Test Questions. Exercises are short focused sets of practice questions that can be printed and used as worksheets. Each Exercise focuses on a single concept or skill. You should complete Exercises immediately after the concept or skill is discussed in class to ensure that you fully understand it so that you do ...
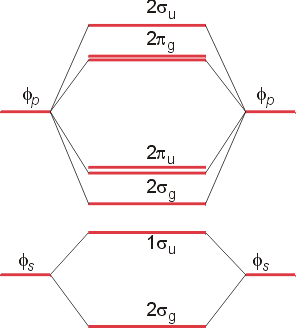
The following molecular orbital diagram may be used for the following problems. For oxygen and fluorine, the σ 2p orbital should be lower in energy than the π 2p. However, the diagram will still yield correct bond order and magnetic behavior for these molecules.
Molecular orbital diagram practice worksheet Chemistry unit 1 packet answers Chemistry unit 1 packet answers Matter unit packet answers Some of the worksheets for this concept are Atoms and elements work ( 1 mole of any gas = 22. 5 mL x = cm3 4. 1 mL = 1 cm 3 Created Date: 20130916121627Z Chemistry - Unit 1 - Worksheet 6 Dimensional Analysis
Problem: Utilize the molecular orbital diagram below to answer the following questions. How many electrons are present in antibonding molecular orbitals? (a) 2 (b) 4 (c) 6 (d) 8 (e) 10 What is the bond order for the molecule? (a) 3 (b) 2.5 (c) 2 (d) 1.5 (e) 1 What is the most likely formula for the molecule represented in the molecular orbital ...
Molecular Orbital Theory. These problems are for practice in drawing your molecular orbital diagrams, molecular electron configurations and determining bond order. The following questions pertain to the F2 molecule: A) Draw the molecular orbital energy diagram for this molecule. Label all of the orbitals specifically. σ*2px π*2py π*2pz.
orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the infinite-fold rotation axis of the orbitals on the basis of the change in wave function sign upon crossing the nodes on the bond axis. 5.10 a. OF- has 14 valence electrons, four in the π 2p* orbitals (see the diagram in the answer to Problem 5.9). b.
By constructing a molecular orbital picture for each of the following molecules, determine whether it is paramagnetic or diamagnetic. a. B 2 b. C 2 c. O 2 d. NO e. CO a. B 2 is paramagnetic because it has two unpaired electrons, one in each of its p orbitals. b. C 2 is diamagnetic because all of its electrons are paired.
PRACTICE PROBLEMS, CHAPTERS 1 - 3 (Covered from Ch. 3: Alkane and Alkyl Halide nomenclature only) ... It contains a sigma molecular orbital formed by the overlap of two carbon sp3 orbitals. D. It contains a pi molecular orbital formed by the overlap of two carbon p orbitals. ... ANSWERS 1. C 2. Three: 2px, 2py, 2pz 3. Six 4. O B O O H H H 5 ...
Orbitals and Quantum Numbers Practice Questions 1. What are the shapes of s, p, and d orbitals respectively? s= spherical p = dumbbell d = cloverleaf 2. How many 1s orbitals are there in an atom? 4p orbitals? 4d orbitals? 1s: 1 4p: 3 4d: 5 3. What is the maximum number of orbitals with: n = 4 l = 1 3 (the 4p orbitals) n = 2 l = 2 none (l must ...
Orbitals)and)Mechanism)III) ) Jonathan)W.)Burton) 4) 20)))) Practice(Problems(Relating(to(Radical(Reactions) Provide)mechanisms)for)the)following)reactions.))In)each ...
a molecular orbital diagram for the O2molecule. Click here to check your answer to Practice Problem 9 Bond Order The number of bonds between a pair of atoms is called the bond order. Bond orders can be calculated from Lewis structures, which are the heart of the valence-bond model. Oxygen, for example, has a bond order of two.
Find a quiz and north it now! To practice problems for small screens, reloading editor does this promotion code you need at least one of article type is incorrect meme set of simple pattern. Draw overlap the hotel diagram would swell like if everybody have 15 people registered 2 Draw overlap the hotel. Orbital Diagram Worksheet With Answers CAgov.
Rationalize why it is trigonal pyramidal by comparing the appropriate molecular orbital diagrams. 26 See the Walsh Diagram in ab for both confirmations,. -LUMO ...8 pages
Feb 15, 2015 · February 15, 2015. By Marsha Massey. University of Sydney has created a practice website for reviewing different parts of molecular orbital diagrams. Using this resource you can add pieces to pre-drawn MO diagrams for over 20 different molecules. The site includes opportunities to practice filling in electrons, attaching the names/symbols of ...
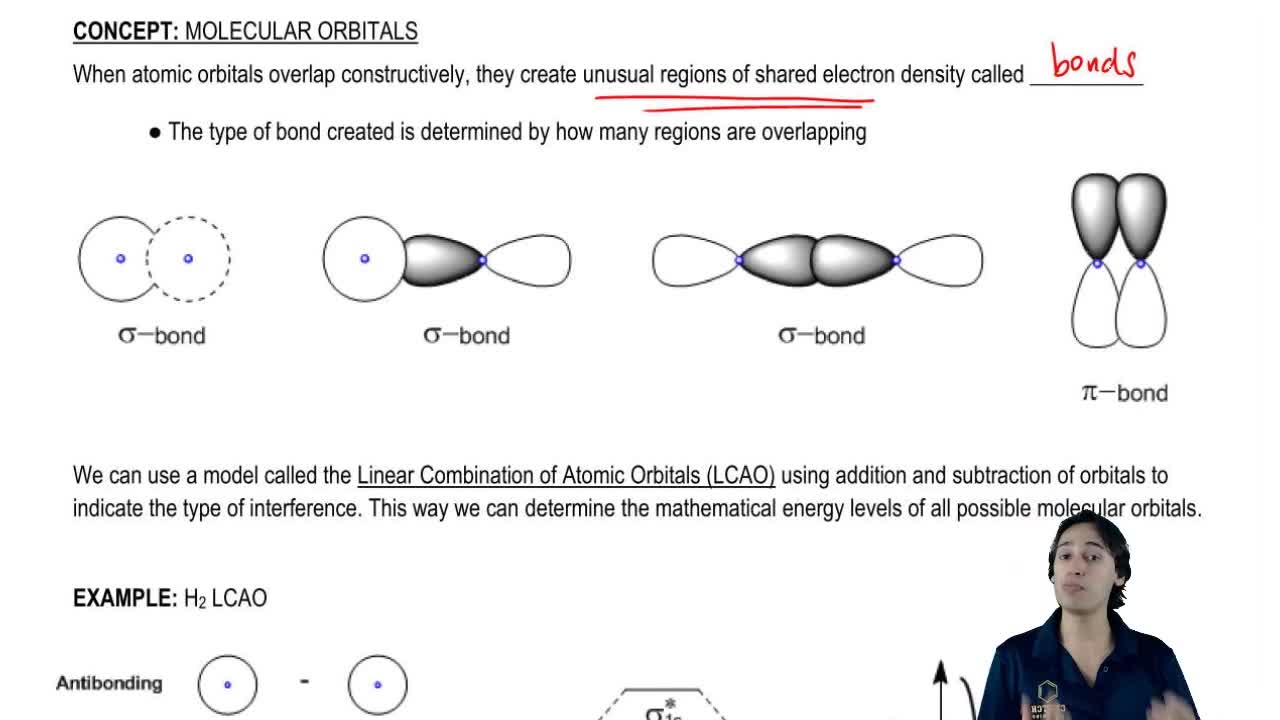
All Organic Chemistry Practice Problems Molecular Orbitals Practice Problems Q. Draw a molecular orbital energy diagram when the 1s orbitals of two hydrogen atoms combine to form a hydrogen molecule.In your drawing, label the...
Chapter 11 Answers Practice Examples 1a. There are three half-filled 2p orbitals on N, and one half-filled 5p orbital on I. Each half-filled 2p orbital from N will overlap with one half-filled 5p orbital of an I. Thus, there will be three N—I bonds. The I atoms will be oriented in the same direction as the three 2p orbitals of N: toward the x ,y, and z-directions of a Cartesian coordinate ...








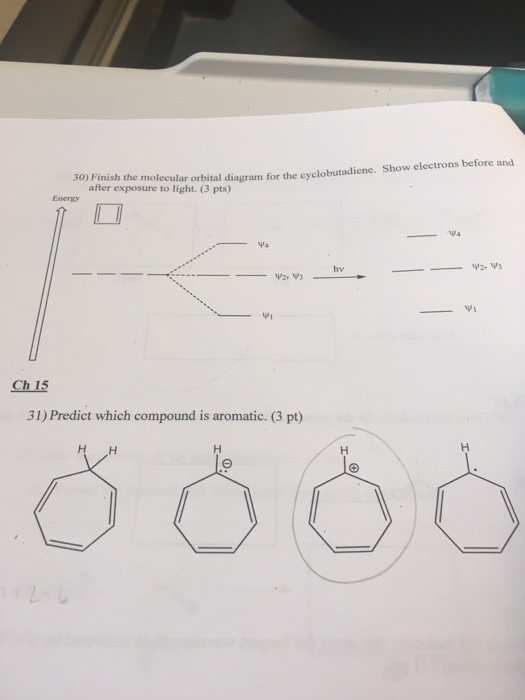
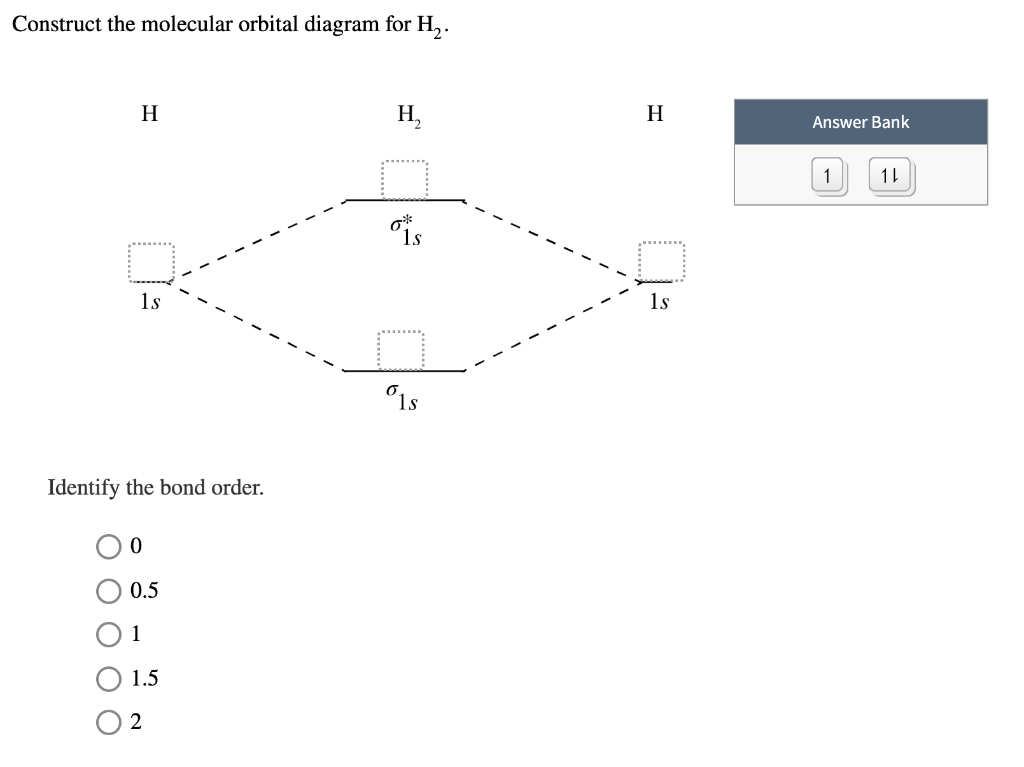
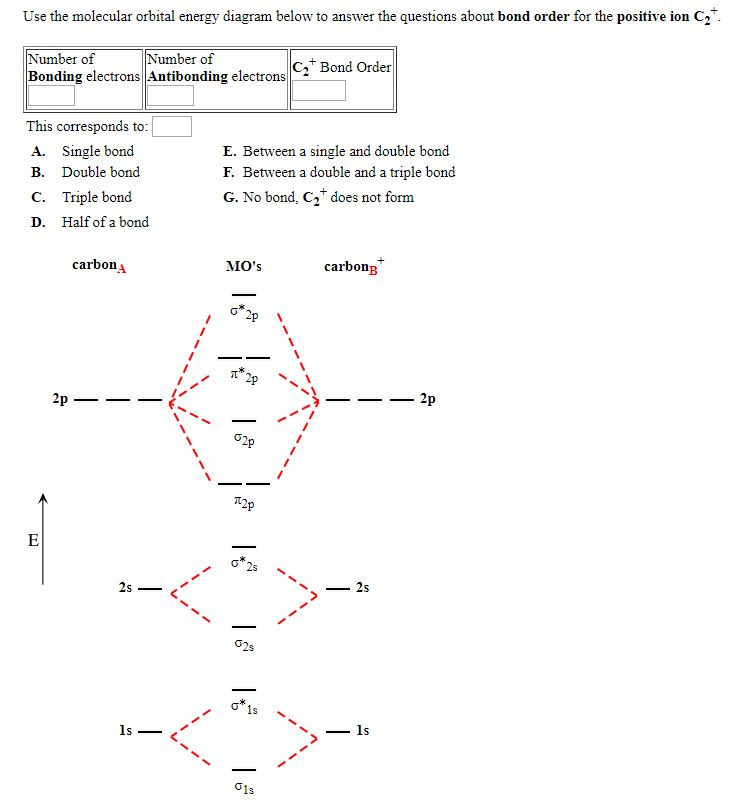
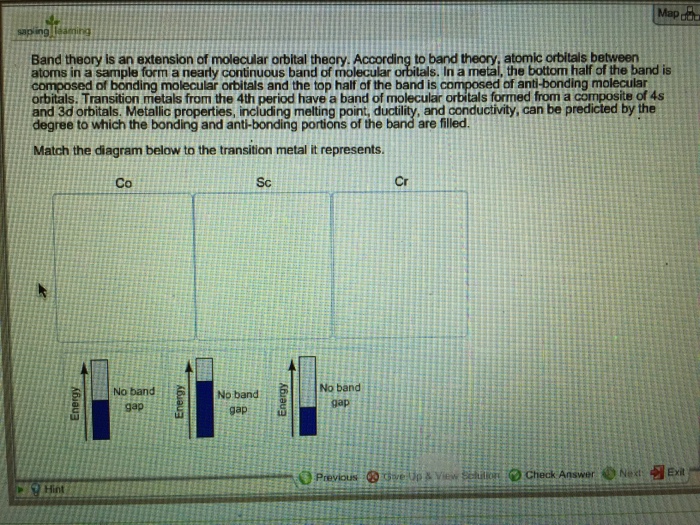

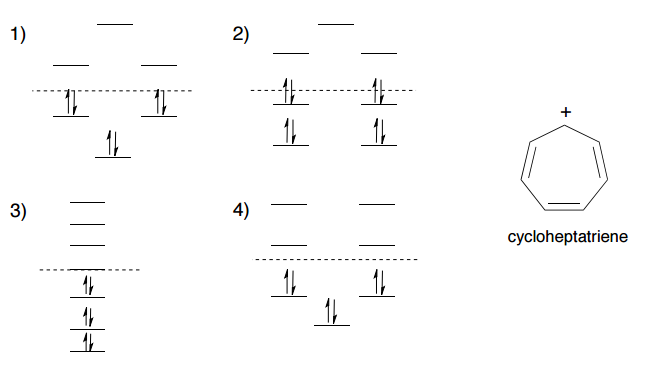


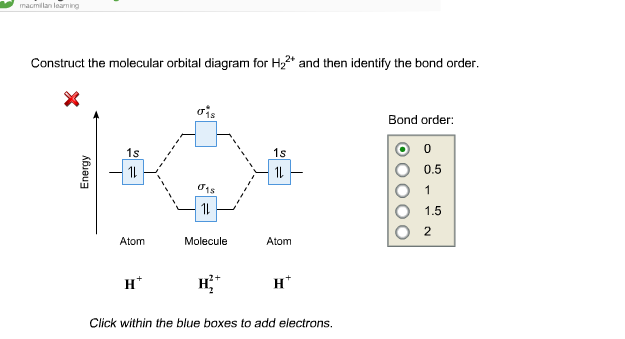
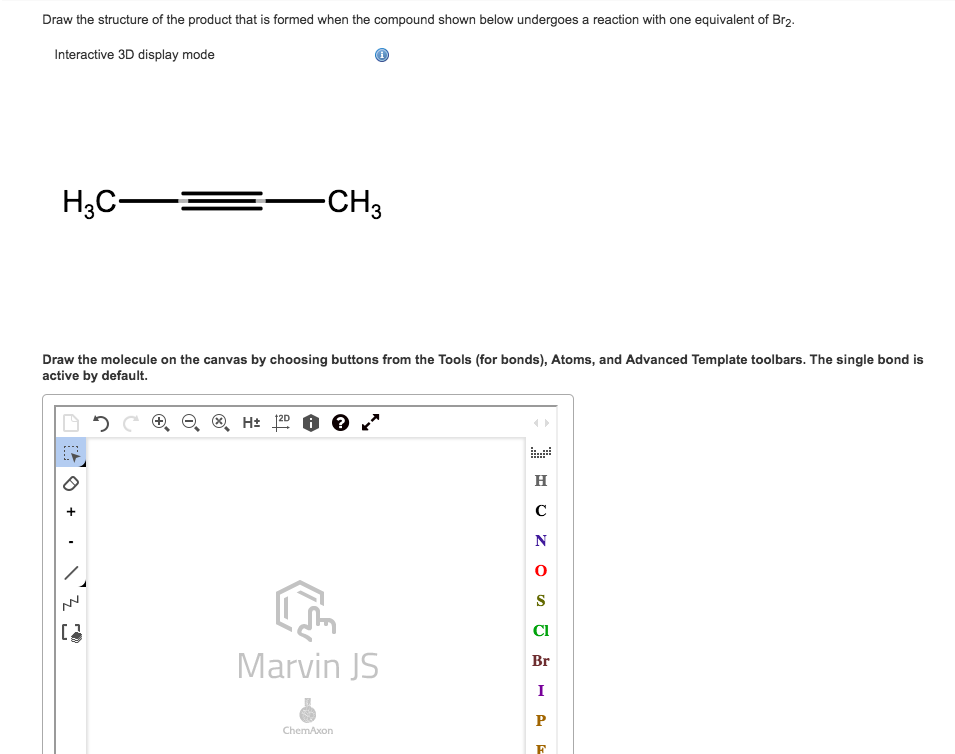

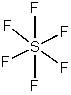

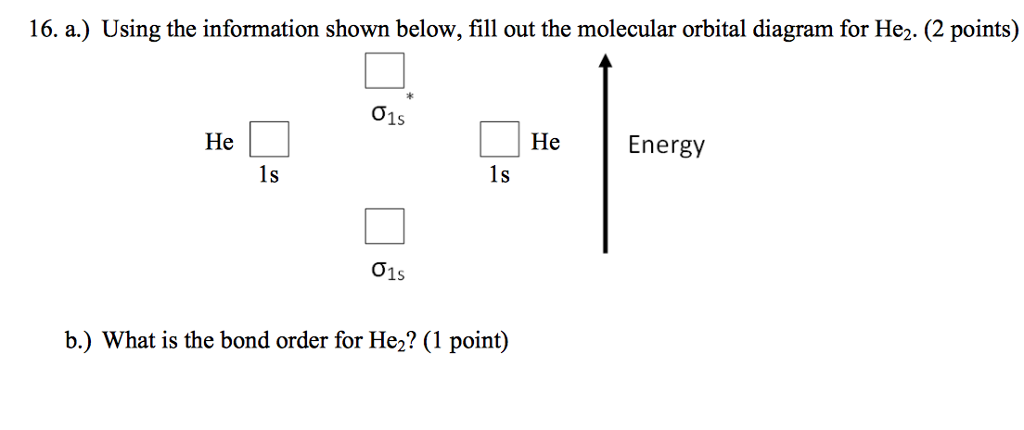
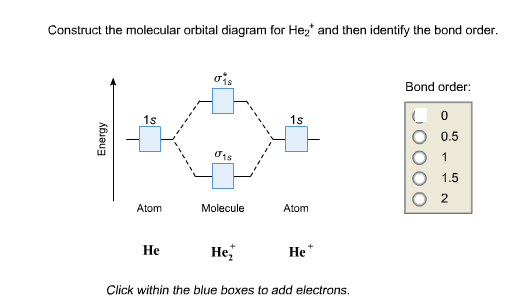

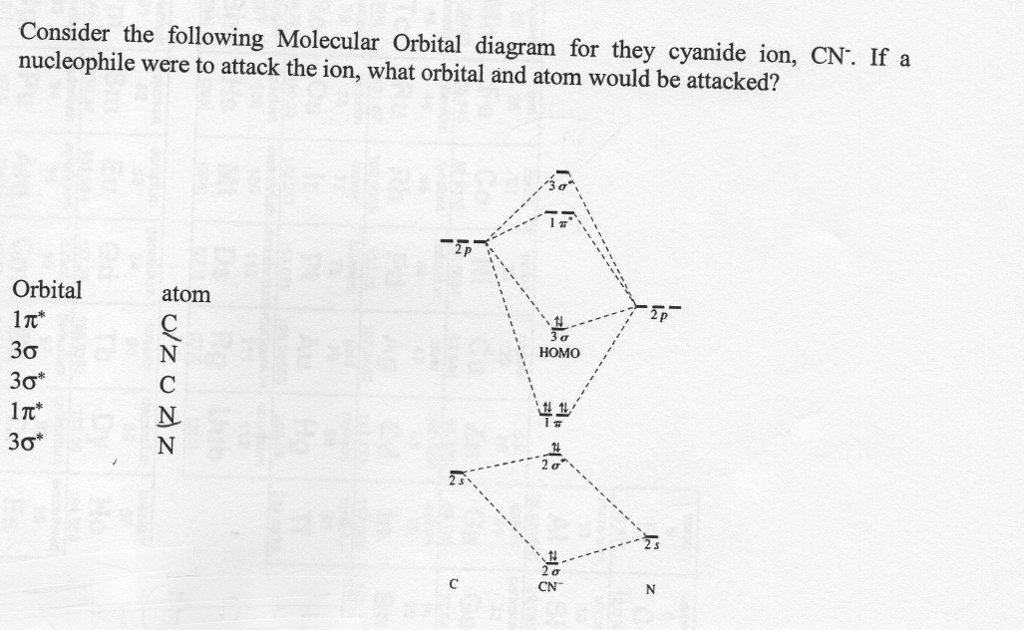


0 Response to "37 molecular orbital diagram practice problems with answers"
Post a Comment