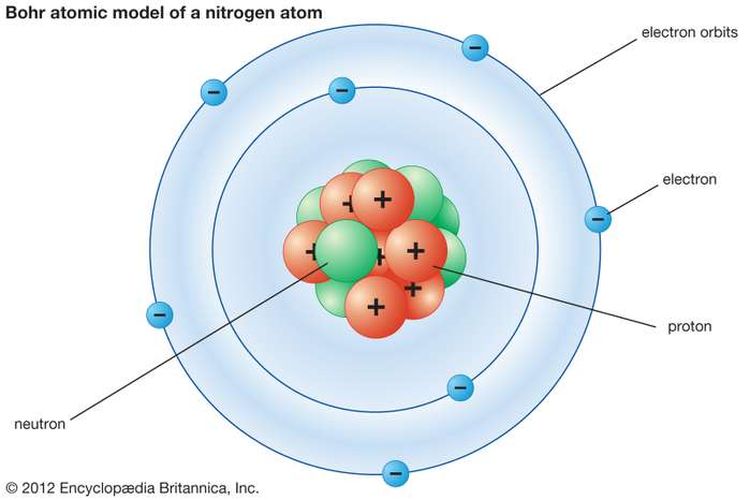
41 bohr diagram for neon
Andrew's model of a neon atom. Find this Pin and more on School projects by Kelly Blankenship. Science Projects For Kids. Science For Kids. School Projects. Chemistry Projects. Neon Atom Model. Atom Model Project. Bohr Model. A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels.
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
Bohr diagram for neon
Neon Bohr Diagram. Neon Bohr Diagram. This phenomenon is really rather puzzling. Essentially, Bohrs model says that electrons different levels of energy depending on how far they have travelled around orbit. Bohr model of the atom explained. why matter is stable: Once an atom is in its ground. Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, well show a sample Bohr diagram for hydrogen. H Hydrogen. 0 neutrons. You can see the principles outlined in the section above at work in the Bohr model for the hydrogen atom. Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons
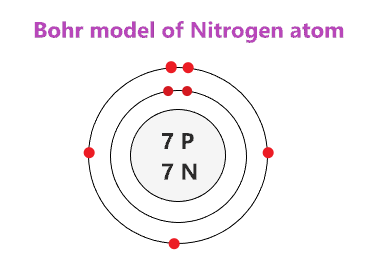
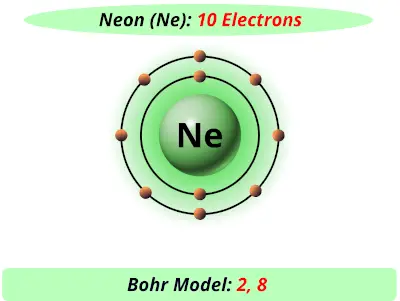
Bohr diagram for neon. Bohr Models and Lewis Dot DRAFT. 10 months ago. by kakins337. Played 219 times ... This is a correct dot diagram for neon (Ne) answer choices . True. False. Tags: Question 12 . SURVEY . 300 seconds . Q. This is a correct dot diagram for carbon (C) answer choices . true. false. Tags: Question 13 . SURVEY . 30 seconds . Q. What is the goal of the ... Write the Lewis diagrams of the following elements, representative of the groups. Note that every member of the group will have the _____ Lewis diagram. Lithium Beryllium Boron Carbon. Nitrogen Oxygen Fluorine Neon. Bohr Diagrams of the Atom. Atoms can be represented by . Bohr. diagrams. Bohr diagrams. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of ...
15 Neon Bohr Diagram. A bohr diagram is a simplified visual representation of an atom that was developed by danish physicist niels bohr in 1913. This is a model that can be used to predict the emission spectrum of a neon. 1 st energy level can hold 2 electrons 2 nd energy level can. Last class, we determined that the Bohr Model is a planetary model in which the For example, there are 3 shells in the bohr diagram of Argon. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer. Bohr model of noble gases, Neon, Argon, Krypton. Bohr Model of Hydrogen. The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. The number of protons in. Bohr Model And Lewis Dot Diagram Worksheet Answers Beautiful The Chemistry Worksheets Atomic Structure Bohr Model. Remember that the maximum number of electrons in the first three shells is 2 8 and 8 Number of electrons 10 10 10 14 18 18 Number of electron shells Atomion neon atom fluorine atom.
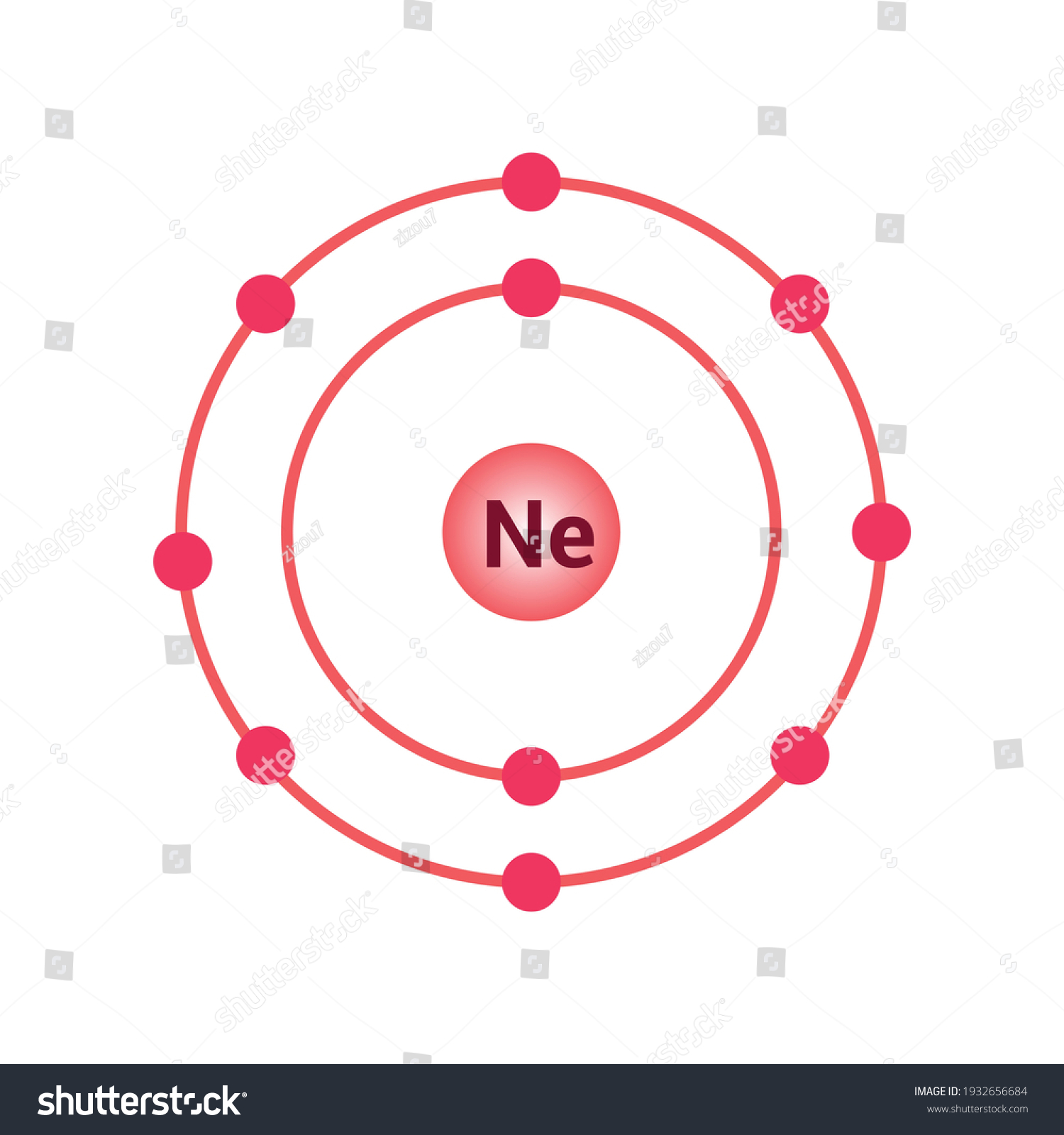
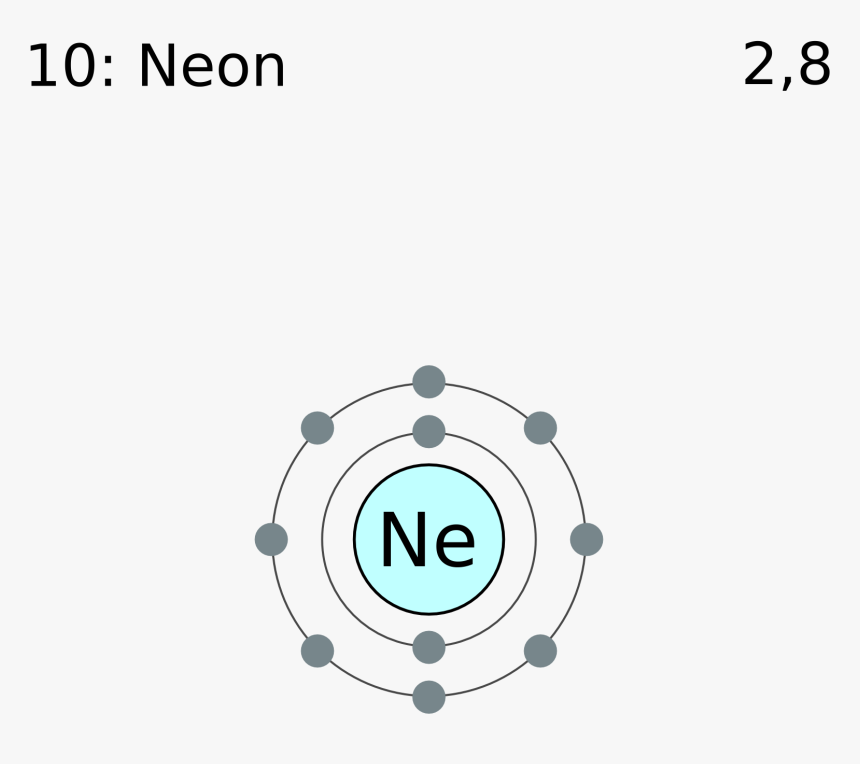
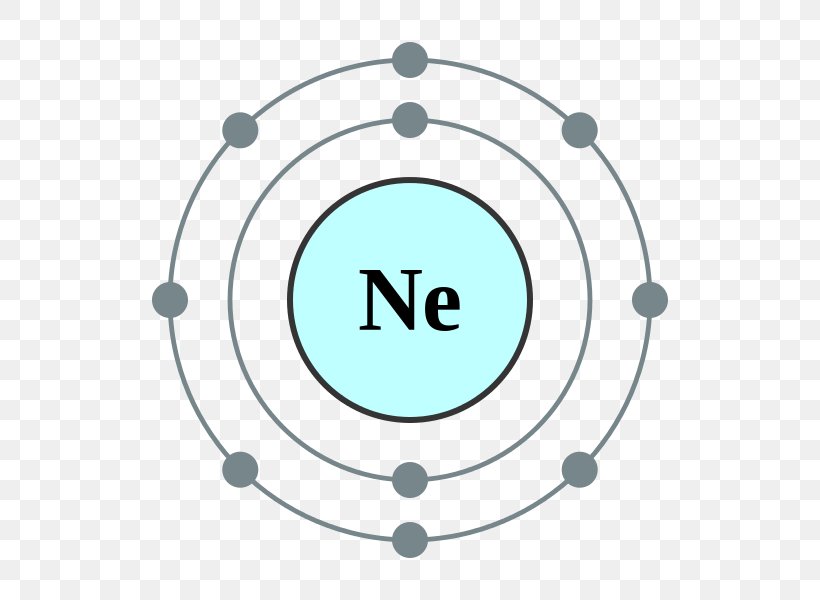
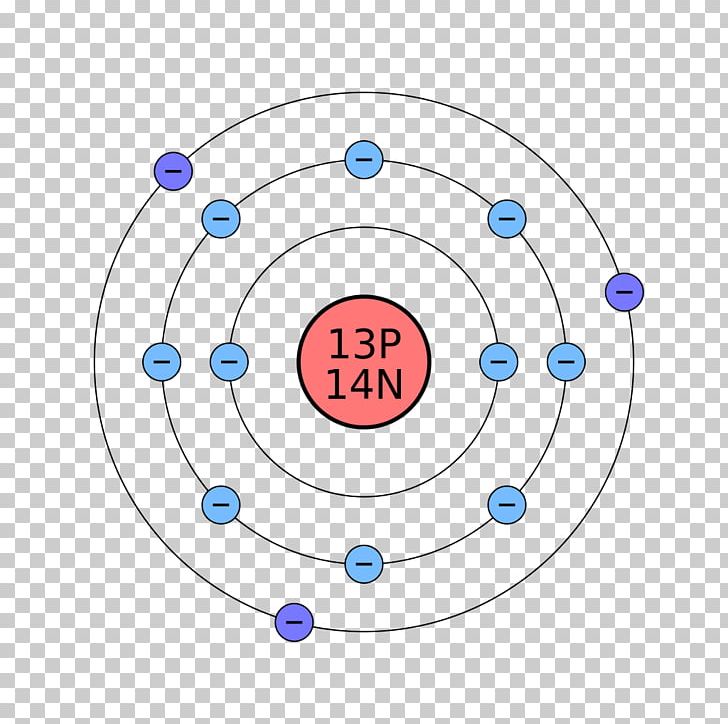
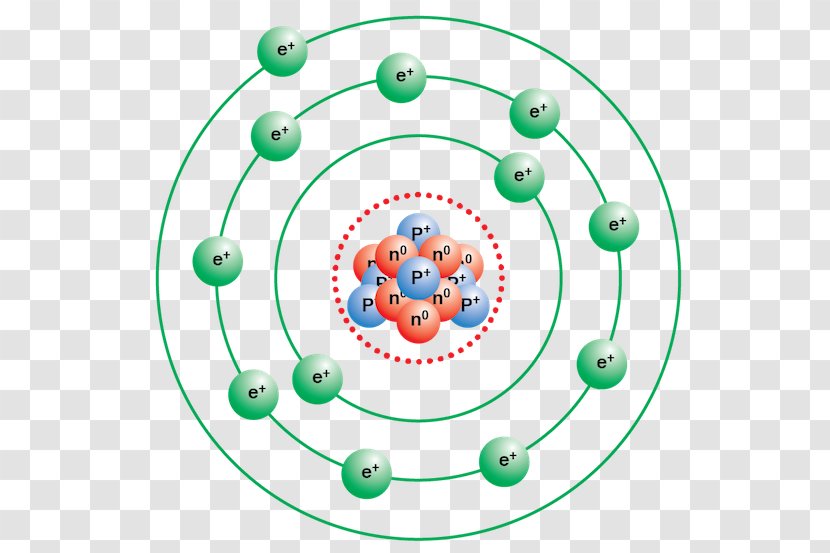
Neon Bohr Diagram. To confirm the third electron of neon enters the orbit of two According to the Bohr hydrogen-like model, the radius. Two electron shells surrounding the nucleus, containing 2 electrons in the n=1 shell and 8 electrons in the n=2 shell. Bohr's model of the atom. Bohr model Neon atomic orbits. Neon has 2 electrons in its first shell and 8 in its secondCheck me out: http://www.chemistnate.com The Bohr Model of Potassium(K) has a nucleus that contains 20 neutrons and 19 protons. This nucleus is surrounded by four-electron shells named K-shell, L-shell, M-shell, and N-shell. The outermost shell in the Bohr diagram of Potassium contains only 1 electron that also called valence electron. An Example of the Bohr Rutherford Diagram of Neon (Ne) The first shell can hold up to 2 electrons, the second shell can hold up to 8 electrons, and the third shell can hold up to 18 electrons but in this case you will only be learning the first 20 elements so it can hold up to 8 electrons An example of this is the Bohr-Rutherford Diagram for.
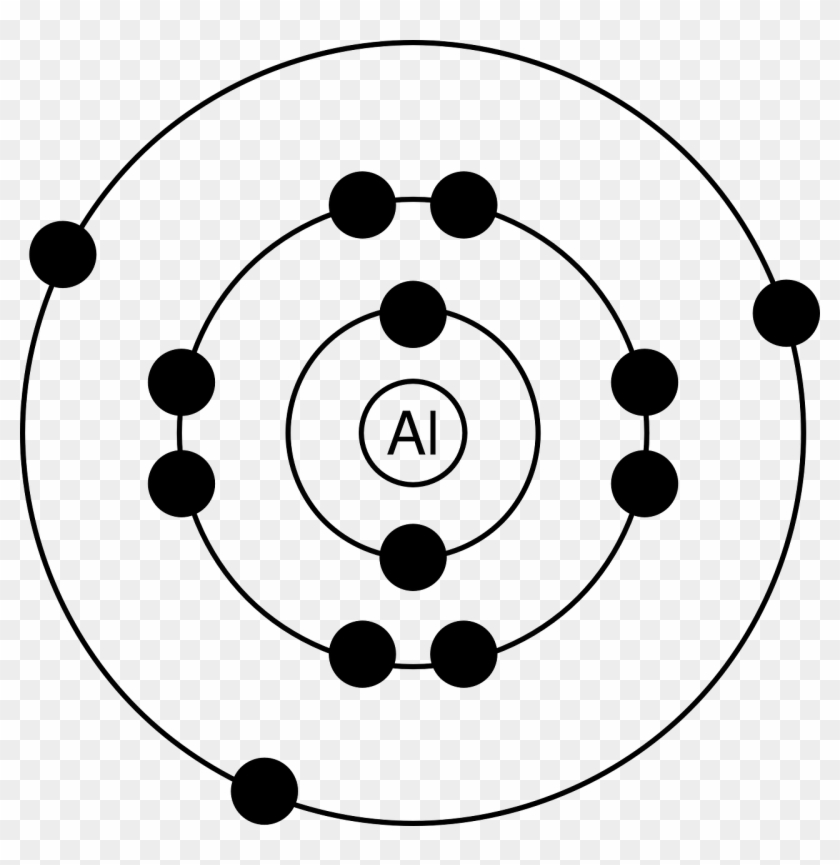
2. Use the table above to draw the Bohr model diagram for the following atoms and ions. Argon atom 22N Chlorine atom Chlorine ion 19 Potassium atom Potassium ion 3. What do you notice about the arrangement of electrons in the Bohr model of a neon atom, fluorine ion, and a magnesium ion? ALI e ec Levels 4.
Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer. Last class, we determined that the Bohr Model is a planetary model in which the For example, there are 3 shells in the bohr diagram of Argon.
Bohr Model of Neon Neon Atom Model, Atom Model Project, Bohr Model, Neon. Visit . Andrew's model of a neon atom Science Projects For Kids, Science. In atomic physics, the Rutherford-Bohr model or Bohr model or Bohr diagram, presented by The second orbit allows eight electrons, and when it is full the atom is neon, again inert.
Oct 29, 2019 - Explore Paula Smith's board "Neon atom" on Pinterest. See more ideas about neon atom, atom, atom project.
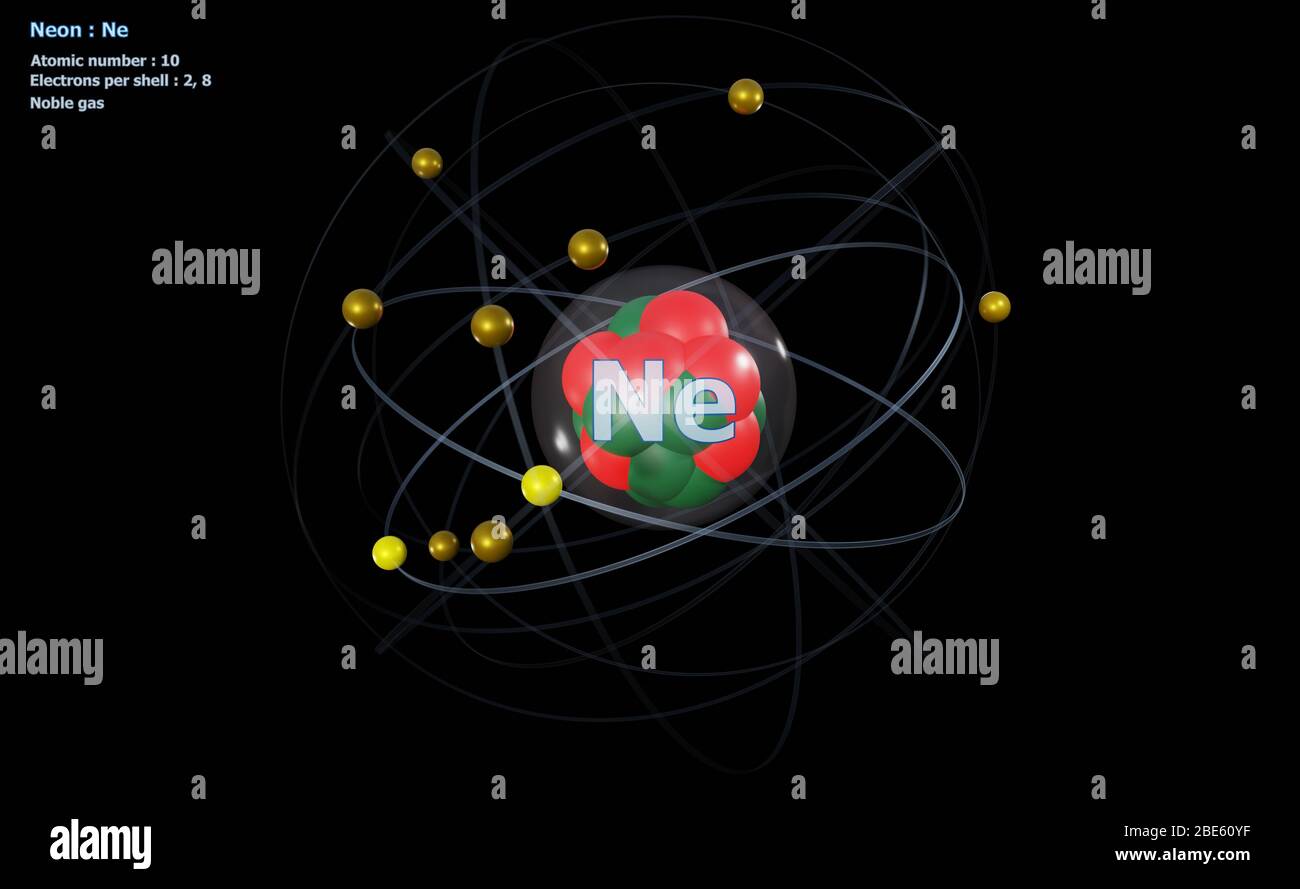
The Bohr Model of Neon(Ne) has a nucleus that contains 10 neutrons and 10 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Neon contains 8 electrons that also called valence electrons.
Bohr Model. Valence electrons: 8. Electron configuration of a neutral neon atom: 1s 2 2s 2 2p 6. Atomic number: 10. Atomic Mass: 20.1797 amu. Oxidation state: 0. Most Common Isotopes (mass number; number of neutrons): 20Ne (20; 10)
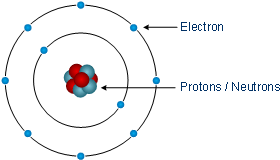
The Bohr Diagram. The Bohr Diagram is what scientists use to explain and show an atom's subatomic particles. This technique was created by two scientists in 1913. They are: Niels Bohr and Ernest Rutherford. [14] This drawing is very simple and easy to do. The number of outer shells an atom has is the number of circles drawn. (Example on page 3).
The Bohr model for neon shows a nucleus with ten protons and neutrons, with ten electrons orbiting the nucleus in two different energy levels.
Bohr Diagrams 1) Add the electrons. 2) Carbon has 6 electrons. C 3) The first shell can only hold 2 electrons. 9. Bohr Diagrams 1) Since you have 2 electrons already drawn, you need to add 4 more. C 2) These go in the 2nd shell. 3) Add one at a time -starting on the right side and going counter clock-wise. 10.
In atomic physics, the Bohr model or Rutherford-Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity.After the solar system Joseph Larmor model (1897), the cubical model (1902 ...
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, well show a sample Bohr diagram for hydrogen. H Hydrogen. 0 neutrons. You can see the principles outlined in the section above at work in the Bohr model for the hydrogen atom.
Neon Bohr Diagram. Neon Bohr Diagram. This phenomenon is really rather puzzling. Essentially, Bohrs model says that electrons different levels of energy depending on how far they have travelled around orbit. Bohr model of the atom explained. why matter is stable: Once an atom is in its ground.





















0 Response to "41 bohr diagram for neon"
Post a Comment