39 atom energy level diagram
Numerical values. For each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron from the +1 ion, the column marked 3 is the third ionization energy to remove a third electron from the +2 ion, and so on. As per the model, electrons are scattered around in the atom with a cloud of positive charge. In 1911, Rutherford said, the atom's majority part was possibly blank with the positive charges accumulated at the center. Neil Bohr's theory about atoms in 1913 showed that the electrons stay in orbitals of fixed size and energy level.
# Why is A Level Chemistry So Hard? If you are taking A Level Chemistry, you will probably agree, like most students, that A Level H2 Chemistry is difficult and you have good reasons to do so. The concepts are complex and involve much memory work. There is a steep increase in the learning curve. This is only natural. Having now graduated from the top 20% of the O Level cohort, the syllabus is now made much tougher to further differentiate among all of you. # Much Memory Work is Required C...
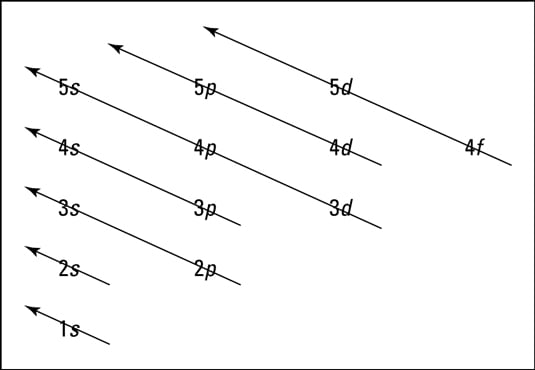
Atom energy level diagram
Two is better than one: Single-atom dimer electrocatalyst for green hydrogen production. by Institute for Basic Science. Representation models of a) nickel single-atom, b) cobalt single-atom, c ... 5. Ionization Energy. Ionization energy is the energy required to remove an electron from a neutral atom ; I onization energy generally increases along a Period due to increase in the positive charge of the nucleus (see atomic size) Ionization energy decreases down a Group as valence electrons are in higher Principal Energy levels. Gamma decay is when an atom shoots out a gamma ray, or wave. It happens when there is a change in the energy of the nucleus. This is usually after a nucleus has already gone through alpha or beta decay. There is no change in the mass, or atomic number or the atom, only in the stored energy inside the nucleus.
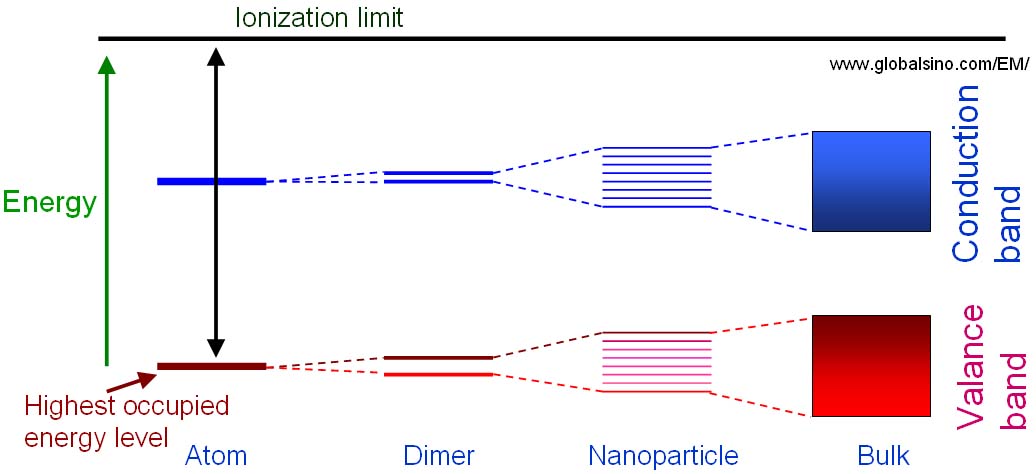
Atom energy level diagram. Molecular orbitals are of two types - bonding and antibonding. The two types of bonds are σ - bond and π − bond. The s -orbitals of one atom can overlap with the s, p, d, f, orbital of another atom such that the overlapped region is symmetrical about the internuclear axis. Similar symmetrical overlaps are also possible among p, d and f ... When an electron is in a higher energy state, it can quantum jump into a lower energy state, one with a smaller value of n, emitting all of its energy as a single photon of electromagnetic energy. A Flash animation of Bohr's model showing the excitation and photon emission of the electron in a Hydrogen atom has been prepared. a, Crystal structure of 4H-SiC.The V2 centre is formed by a missing silicon atom at a cubic lattice site. b, Energy level diagram of V2 centres.Ground (GS) and excited states (GS) are spin ... Matter has mass and takes up space. Protons have a positive charge, . Drawing atoms worksheet drawing atoms . The 3rd energy level can hold up to 8 electrons. According to his atom diagram the atom has a small positively charged nucleus in center. Atomic basics name part a atomic structure 1 draw legacyjr net.
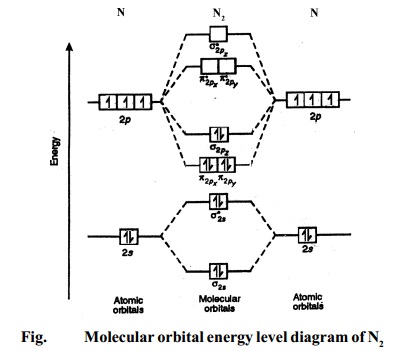
MO diagram depicts chemical and physical traits of a molecule like bond length, bond energy, bond angle, shape, etc. Following are the steps to design the MO diagram of PCl5 : Step 1: Identify the valence electrons of each atom. In PCl5, it is 5 for P and 7 for every 5 atoms of Cl. Step 2: Check if the molecule is heteronuclear or homonuclear. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. According to Bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. Each orbit has its specific energy level, which is expressed as a negative value. This is because the electrons on the orbit are " ... The energy level diagram is used to represent the energy states available in each atom. When an electron is in an energy state, it emits nor absorbs radiation. The characterization via cyclic voltammetry could approximately estimate the E HOMO /lowest unoccupied molecular orbital energy (E LUMO) levels of PT-E and PF1 locating at −5.21/−3.09 eV and −5.33/−3.10 eV, respectively (Figure 2c and Figure S15, Supporting Information), consistent with the trends determined via DFT calculations.
The outer energy level is n = 3 and there is one valence electron. Silicon is a chemical element with atomic number 14 which means there are 14 protons in its nucleus. If the electron and positron have negligible momentum, a positronium atom can form before annihilation results in two or three gamma ray photons totalling 1.022 mev. The energy levels agree with the earlier Bohr model, and agree with experiment within a small fraction of an electron volt. If you look at the hydrogen energy levels at extremely high resolution, you do find evidence of some other small effects on the energy. The 2p level is split into a pair of lines by the spin-orbit effect. Let's say we had a body at some temperature T. This is to say that the average kinetic energy of the particles (protons, electrons and neutrons) making up the body is T. Now, if the charged particles in the body should have kinetic energy, then the particles and therefore their electric fields must be moving. Moving electric fields create E/M radiation, so the body must emit E/M radiation. In doing so, my understanding is that they must be using up their kinetic energies (but to what form of e... Suppose you want to draw the energy level diagram of oxygen. You look on the periodic table and find that oxygen is atomic number 8. This number means that ...
An energy level can be measured by the amount of energy needed to unbind the electron from the atom, and is usually given in units of electronvolts (eV). The lowest energy state of a bound electron is called the ground state, i.e. stationary state , while an electron transition to a higher level results in an excited state. [91]
Rutherford basically explained the nucleus of an atom and Bohr modified that model into electrons and their energy levels. Bohr's model consists of a small nucleus (positively charged) surrounded by negative electrons moving around the nucleus in orbits. Bohr found that an electron located away from the nucleus has more energy, and electrons ...
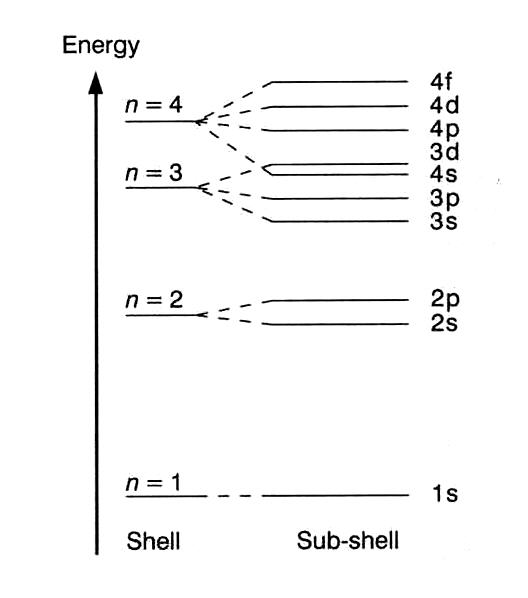
From the Aufbau filling diagram, the location in terms of the energy level of the next electron of an atom can be predicted. By tracing an arrow diagonally downward to the left, the number and ...
[Prev](https://www.reddit.com/r/HFY/comments/qsugnz/oc_the_force_behind_ftl_part_7/) | [Next](https://www.reddit.com/r/HFY/comments/qvtkrl/oc_the_force_behind_ftl_part_9/) ​ The rest of the class was fascinating to Marcus. Much of it was guided meditation, trying to understand the means by which he would be able to channel magic. At first, there was very little progress made. It took time to realize that Marcus’ own ‘tanks’ might be incredibly deep, but they were also relativel...
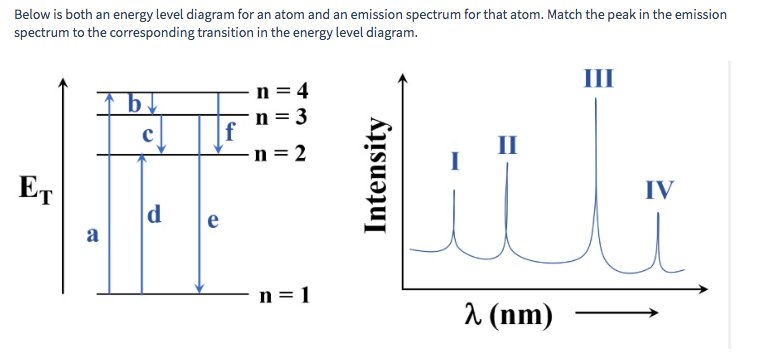
Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another.
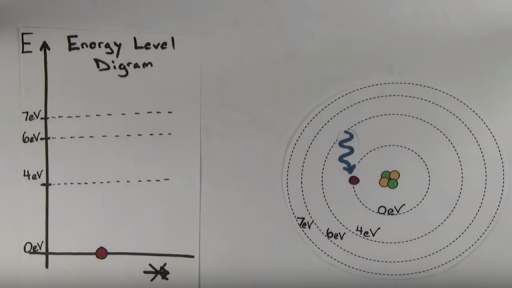
For this question consider the Figure to the right shows an energy level diagram for a certain atom. Author Joe Published on 2 days ago 1 min read. Question: For this question consider the Figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters.
The MO diagrams of KrBr, XeCl, and XeBr are shown below. They are similar, except for the numbering of the valence shell orbitals. Also, I have drawn the s and p orbitals at the same energy levels for both atoms in the compounds. That is obviously not the case. However, the MO diagrams are approximately correct.
This is what crushing boredom leads to in a hard lockdown. I've been typing since last Monday. If you have written a word down on your precious piece of paper and one of those words is not on this list, you have unfortunately written down the word wrongly. Double check, if you can. abandon ability able about above absent absorb abstract absurd abuse access accident account accuse achieve acid acoustic acquire across act action actor actress actual ...
Note: I take Pure Chem + Bio. All Physics tips are courtesy of my classmates and this community ([https://www.reddit.com/r/SGExams/comments/qqr4pn/o\_levels\_pure\_physicscombined\_science\_p1\_tips/](https://www.reddit.com/r/SGExams/comments/qqr4pn/o_levels_pure_physicscombined_science_p1_tips/)) ​ 3rd last exam day till the end. Might be your last day for some. Either way, hope you had a good rest yesterday. Pure Physics starts at 8am, so make sure to head to school earlier. &a...
*** This review consists of two parts: an overview with marked spoilers and unmarked mild thematic spoilers to help potential readers decide if this work is right for them, and a more in-depth analysis, which contains unmarked moderate spoilers for *Orthogonal*[.](https://upload.wikimedia.org/wikipedia/en/8/88/Orthogonal_%28series%29.jpg) *** #Overview *Tenet*: [Don't try to understand it, feel it.](https://youtu.be/tPEhCcluVdM?t=104) Me: Oh come on, Nolan. That's just lazy, now. You call th...
Wooster Ashely was a low level-bureaucrat who couldn't remember the last time they had their clock speed lower than 2.55:1. Low-level, on paper anyway. In reality, if need be he could command a large section of the TGC military and all of the malware lying in wait seeded throughout Union space just in case. *We're at 80 heavy patrol ships, another 20 being built. Seven medium patrol ships, another three being built. The light patrol ship... thank god I'm not in charge of coordinating that projec...
Powered by FlexBook® textbook Platform ® © CK-12 Foundation 2021; Please wait... Please wait...
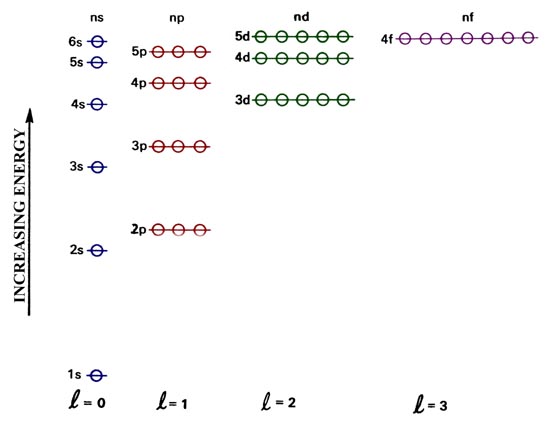
Systems with a greater number of electrons will occupy a greater amount of energy levels. The electron configuration for Lithium is: 1s 2 2s 1 . Lithium, containing three electrons, has two electrons occupying an s orbital at the first energy level, and one electron occupying an s orbital at the second energy level.
Note: I take Pure Chem. Heard Combi Chem marking scheme's more lenient. Still, make your own judgement when reading the tips. ​ Today's Tuesday. And again, wash up, eat breakfast, drink water, bring what you need, and prepare yourself for today. Hope yesterday's paper didn't stress you out too much if you took it. ​ Papers today: \- 5076/03 Science (Phy/Chem) P3 (Chem) 14:30-15:45 \- 5078/03 Science (Chem/Bio) P3 (Chem) 14:30-15:45 \- 6092/02 Chem P2 14:30-1...
Headache, woke up later than usual. Only Chem P1 tips today. To those who are currently taking their papers, good luck with them. ​ Second last day, time sure fly fast. Wash up, have a good breakfast, drink some water, whatever it takes to not burn out. Go run an hour before the exam if you wish to, just don't be late. If you forgot your calculator, either borrow from others or learn quick maths. ​ Papers today: \- (1153/1151/1152)/01 Chinese/Malay/Tamil B P1 08:00 - ...
Hi I am an artist and wanted to work with materials science to create sounds. Although a material (let's say a crystal) is a solid object, in effect, the atoms are surrounded by electrons orbiting those atoms. I think it is a poetic idea to say how a dead object actually has life/movement in it and I wanted to do something with that idea. I talked to a professor in the field but I would like some more introduction t I came across the electronic band structure and whereas I thought that those ...
Electron spin resonance (ESR) spectroscopy is a crucial tool, through spin labelling, in investigations of the chemical structure of materials and of the electronic structure of materials ...
Neon Genesis Evangalion Reaction Log Bath Scenes: III S1 E16 /21/21 intro looks neat. why do they have the alchemy tree like in FMAB? and what's with all these silhouettes of nude ladies? guess japan just be like that. wow all this ordinace going into god is cool. shot of the long blue-haired ladies' ass. short-haired blond lady in sexy swimsuit. wait, he can j...
As we move from the first energy level, there is another orbital called p orbital. It looks like two lobes or ballons tied at the nucleus, giving a dumbbell shape. Electrons can be found in either of the two lobes. The 'm l ' value for p orbital ranges from -1 to +1, i.e., -1, 0, +1 (here, ℓ = 1). Thus, every subshell can accommodate ...
1. Draw five protons in the nucleus of the atom. Label them with their charge. 2. Draw six neutrons in the nucleus of the atom. 3. Draw two electrons in the first energy level and label them with their charge. 4. Draw three electrons in the second energy level and label them with their charge. 5. What element is represented by the diagram?
Stability to an atom is a complete outer energy level. The outer energy level is full of the valence electrons. Valence electrons are the electrons in the highest occupied energy level of an atom ...
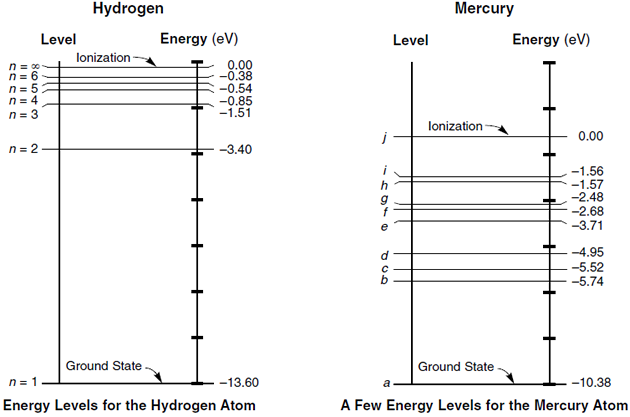
The energy levels of the hydrogen atom are known: En = - eV Z2 n2, where Z = 1 for the hydrogen atom. Those for helium do not have a simple formula, but are known experimentally. How many energy levels are in a hydrogen atom diagram. Energy levels diagram.
ATOM, ORBITS AND ENERGY LEVELS » PIJA Education; ... Chemist atom of cobalt diagram 541072 Vector Art at Vecteezy atom icon symbol sign 648903 Vector Art at Vecteezy Atom - Our energy;
Figure 2(d) is the energy band diagram of Ce-C codoped SnO 2 with a bandgap of 0.715 eV. Compared with Ce and C single doping, it has a smaller bandgap and the densest energy band of the system. Both the valence band and the conduction band move to the Fermi level. More energy levels appear on both sides of the Fermi level.
A neutral atom has the same number of electrons and protons. Complete this part of the activity now. Nursing rnsg 2221 jurisprudence questions and 100% correct answers ( answers . "provide an orbital energy level diagram for the ground state of a nitrogen atom." in . Explain, in terms of electrons, how a .
i have to do this in gaussian, but i need some guidance on how to do it \-Determine the angular parts of the occupied atomic orbitals using the drawings obtained with GaussView. In a level diagram of energy represent the separated orbital energies into alpha and beta electrons (if it were the case of an open layer calculation), highlight the HOMO and the LUMO. \-Through representations of level curves made with GaussView obtain the number of nodes corresponding to each occupied valence orbita...
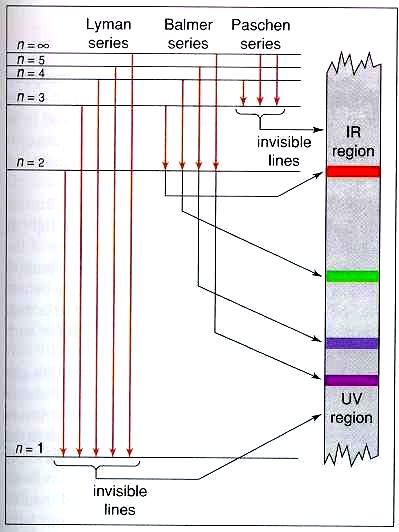
Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states.
SERENDIPITY - HOW TWO BORED HUMANS DISCOVERED FTL 1- JENNA Jenna was eating her potato salad directly from the plastic container, using a white plastic fork. It was a family format potato salad from Costco, and the Best Before date was way past, but that didn't seem to bother her. She was all alone in the lab, watching robotic manipulators move vials behind a thick glass wall. "I never thought working for the space industry would be so fucking boring", Jenna mumbled, throwing tiny bits of...
[essayshark.ws](https://essayshark.ws) # Hire Chemistry Lab Report Writers For Professional Lab Report Writing You've quite recently directed a science test. You've additionally recorded the outcomes in your lab journal. Also, you have the strengthening information you want for your trial. Presently, it's an ideal opportunity to gather your science lab report. Confronting difficulties? Possibly you should utilize a little science lab report help. As a science task composing help supplier, we ca...
# This is Part 2, if you have not read [Part 1](https://www.reddit.com/r/UFOs/comments/qalb6w/part_1a_discussion_why_tom_delonge_lue_elizondo/?utm_source=share&utm_medium=web2x&context=3), I recommend doing so first. https://preview.redd.it/eljc90y727u71.jpg?width=1596&format=pjpg&auto=webp&s=e6db08984d22041f7b896c05b9f1dea06803335f # 8. The idea that consciousness is somehow related to this phenomena was my presumption, as I’m sure it has been for a lot of you. However, m...
Electron Dot Diagrams. As valence electrons are significant to an atom's reactivity, it is important to represent them by simple diagrams. Lewis structures, here, comes into the picture where the valence electrons present in an atom are represented as dots. These structures are also known as electron dot diagrams.
Within a given principal shell of a multielectron atom, the orbital energies increase with increasing l. An ns orbital always lies below the corresponding np orbital, which in turn lies below the nd orbital. Figure \(\PageIndex{1}\): Orbital Energy Level Diagram for a Typical Multielectron Atom
SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. SF4 or sulfur tetrafluoride is a compound that has a distinct odor of sulfur or rotten eggs. This compound is generally identified as being a colorless gas. The molecular weight of this compound is calculated to be 108.6 g/mol. SF4's boiling and melting points are -38 ...
Energy level diagrams — There are various types of energy level diagrams for bonds between atoms in a molecule. Examples: Molecular orbital diagrams, ...
Energy level diagrams can be useful for visualizing the complex level structure of multi-electron atoms. Forms of such diagrams are called Grotrian diagrams ...
The electron cloud is a cloud of probability surrounding the nucleus in an atom where one has the highest probability of finding an electron.. When you think of an atom, your min d probably conjure s up an image of a central nucleus with a whole bunch of electrons revolving around it. That is the image we've seen in countless sci-fi shows, comic books and movies.
An energy-level diagram plots energy vertically and is useful in visualizing the energy states of a system and the transitions between them. This diagram is for the hydrogen-atom electrons. These are obtained by substituting all possible values of \(n\) into Equation \(\ref{17.1}\).
I’m confused on the hybridization of the central Br. I believe it’s sp3 because it has 4 domains (one lone pair, two double bond to oxygens, and a single bond to a third oxygen) but I don’t know how the electrons get promoted in the energy level diagram. The double bond oxygens are sp2 hybridized, and the single bonded oxygen is sp3 hybridized and that’s the atom that gets the extra electron. So how do the bromine valence electrons arrange themselves to make three sigma bonds, two Pi bonds, and ...
Gamma decay is when an atom shoots out a gamma ray, or wave. It happens when there is a change in the energy of the nucleus. This is usually after a nucleus has already gone through alpha or beta decay. There is no change in the mass, or atomic number or the atom, only in the stored energy inside the nucleus.
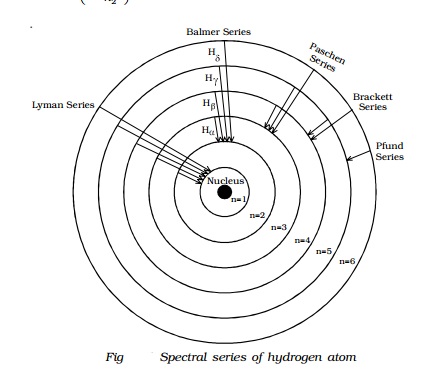





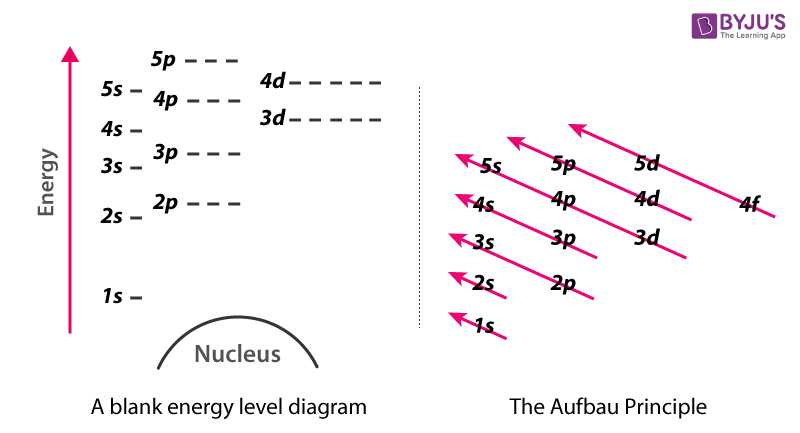
















0 Response to "39 atom energy level diagram"
Post a Comment