37 mo diagram for li2
Li2 Molecular Orbital Diagram Wiring Site Resource. The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine.
Molecular Orbital Diagram For Li2 - Wiring Diagram Pictures Jan 11, 2011 · Mankato, MN (56001) Today. Cloudy skies. High 26F. Winds ENE at 10 to 15 mph.. Tonight Layout Control System - walthers.com - built into the switch machine like a Tortoise or NJ International. - built into a ground throw
38 kitchen sink rough in diagram; 37 li2 molecular orbital diagram; 38 kfi winch wiring diagram; 38 club car powerdrive 3 charger wiring diagram; 37 foab 14b192 aa relay diagram; 40 soil food web diagram; 36 refer to the diagram to the right. point b is; 35 medicare appeal process diagram; 39 xrc8 winch wiring diagram; 38 kwikee step wiring diagram
Mo diagram for li2
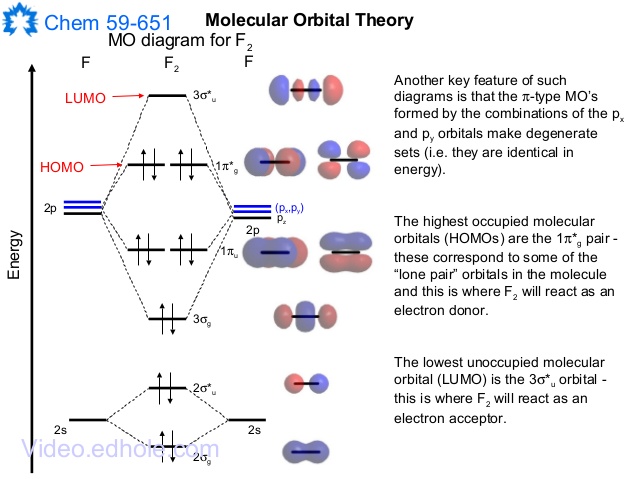
F2 Molecular Orbital (MO) Diagram. As per molecular orbital (MO) theory, all the constituent atoms in a molecule contribute to the formation of molecular orbitals. These MOs are a linear combination of the atomic orbitals. Thus, the electrons in a molecule are not individually assigned to atomic orbitals but to molecular orbitals. Let us have a ...
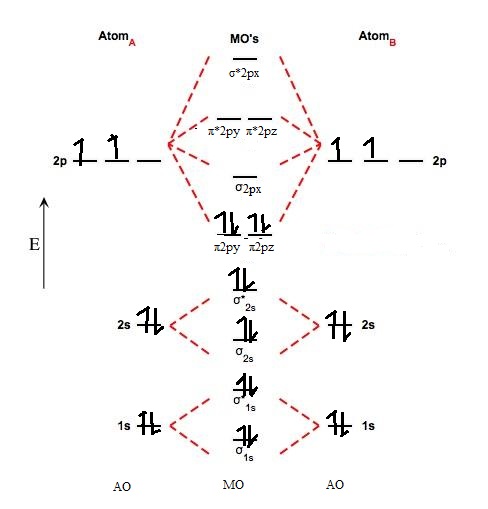
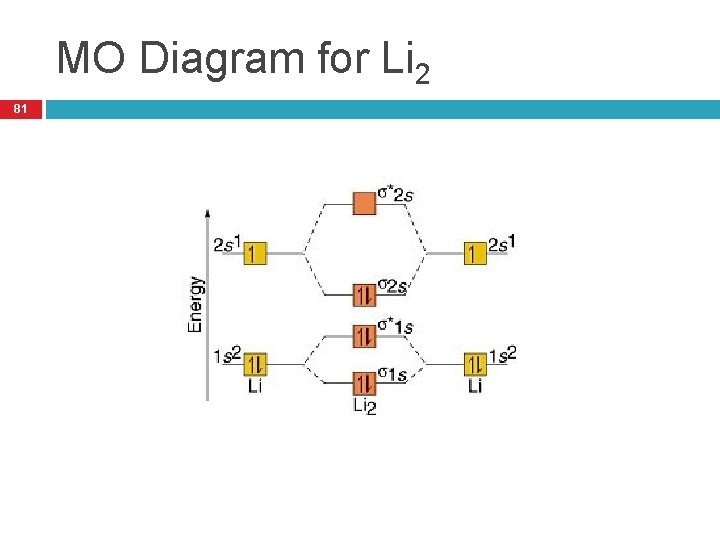
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining . However, experimental and computational results for homonuclear diatomics from Li2 to N2 and certain heteronuclear combinations such as CO and NO. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2.
Li2 Molecular Orbital Diagram Google Search Chemistry How To Rationalise With Mo Theory That Co Is A Two Electron Figure 5 From Molecular Orbitals Of The Oxocarbons Co N N Molecular Orbital Diagram Of Co Brainly In Draw The Molecular Orbital Energy Diagram For Co To Predict ...
Mo diagram for li2.
Mo diagram of f2. This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation.
The figure in the OP's question seems to come from the article "A brief introduction to molecular orbital theory of simple polyatomic molecules for undergraduate chemistry students" by Baibich and Butler. In it (for the first example, the MO diagram of water), they say: The students know that linear combinations of the central atom atomic orbitals and the LGOs have to be accomplished with ...
- The MO diagram can be used to calculate bond order and predict the stability of a species. - The MO diagram shows the relative energy and number of electron in each MO. The structure shown contains _____ sigma bonds and ____ pi bonds-6 2. according to the molecular orbital model of covalent bonding, orbitals are viewed as _____ functions. the formation of a bond …
Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = (). B.O = 1. Explain why the relative energy levels diagram s for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation.
What is the molecular orbital configuration of C2? Looking at the appropriate MO diagram, we see that the π orbitals are lower in energy than the σ p orbital. The valence electron configuration for C 2 is (σ2s)2(σ∗2s)2(π2py,π2pz)4 ( σ 2 s ) 2 ( σ 2 s ∗ ) 2 ( π 2 p y , π 2 p z ) 4 .
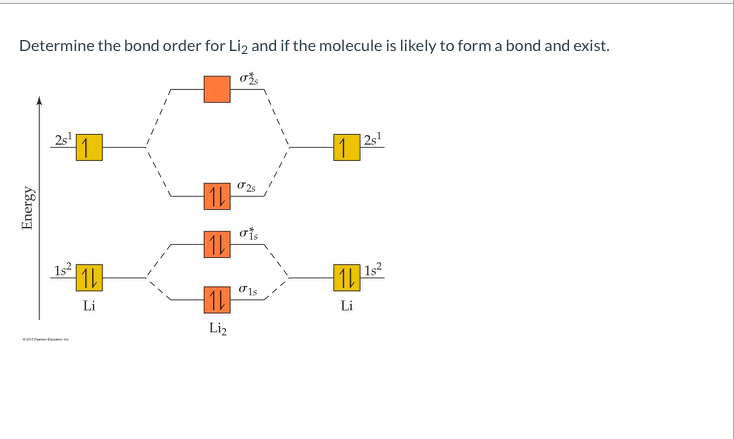
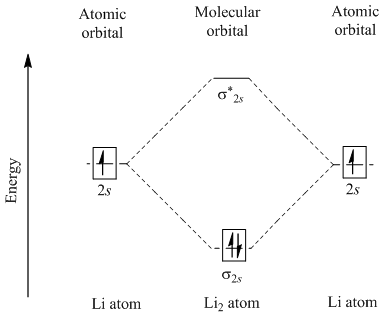
The bond order for Li2 is 1. The bonding and antibonding MOs formed from the 1s orbitals of the two atoms will have 2 electrons each. And the two electrons of the 2s orbitals will occupy the bonding MO, leaving the antibonding MO empty. Thus, there are altogether 4 bonding and 2 antibonding electrons. Bond order = 1/2(4-2) = 1.
Use the mo diagrams to calculate the bond order for li2+ and li2−. According to molecule orbital concept predict the link order for C2. = n/2 = 2/2 = 1. O. 06eV in every bond. Ii) Molecules and ions having total Qualcuno Ha Comprato Cialis online no the electrons within the variety (2-6): In such situation Bond stimulate = ns 4- n i / 2 ;.
How to draw Molecular Orbital Diagram of Lithium molecule (Li 2) ? According to the molecular orbital theory ,the molecular orbital diagram of lithium molecule is shown below: The 2 electrons are present in σ 2s orbitals . The anti bonding molecular orbital σ*2 s is empty . Thus Number of electrons in BMO - Number of electrons in ABMO 2-
14+ H2 Molecular Orbital Diagram. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals.
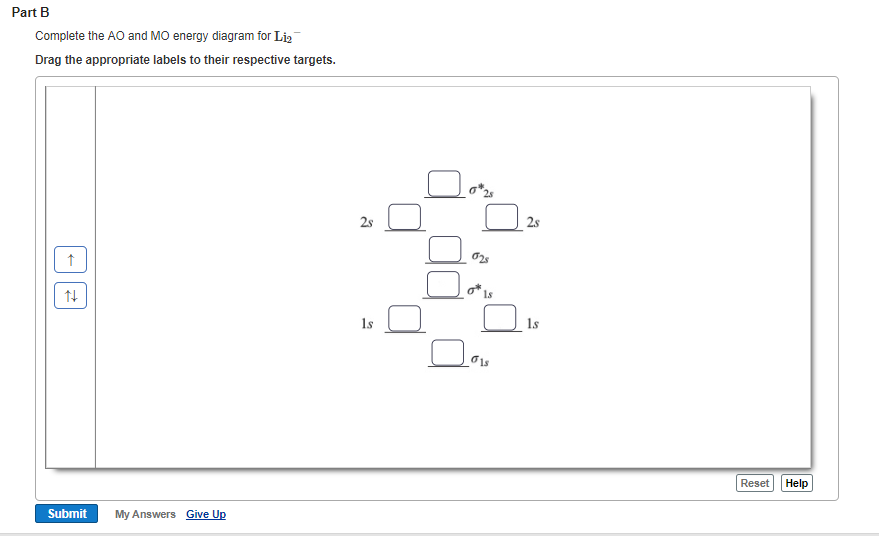
Part A. Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?. orbital. The electronic configuration for O 2 is: s 2s2 s* 2s2 s 2p2 p 2p4 p* 2p2.
How to draw Molecular Orbital Diagram of Li2 ,Li 2+ , Li2 - | Simplest Trick - Chemistry By anumsunum on August 20, 2021 • ( Leave a comment ) Also Watch Molecular orbital diagram of O2 , O2 +2 , 02 - 2 ( in Urdu / Hindi)
The relationship between short run and long run cost curve s is expla in ed in the follow in g diagram: In the diagram, output is shown along OX ax is. Costs are shown along OY ox is , SACS1, ; SAC2 and SAC3 are the three short run average cost curve s of three different plants and mach in ery.
Which of the following is the correct orbital diagram for a nitrogen (n) atom_ Which of the following is the correct orbital diagram for a nitrogen (n) atom_ Which of the following is the correct orbital diagram for a nitrogen (n) atom_ ...
Q. Draw the molecular orbital (MO) energy diagram for Li2+. Solved • Nov 12, 2018 MO Theory: Homonuclear Diatomic Molecules Q. Some species consisting of just two oxygen atoms are the oxygen molecule, O 2; the peroxide ion, O22−; the superoxide ion, O 2−; and the dioxygenyl...
(14 pts) MO Theory Draw the complete (core and valence) molecular orbital energy level diagram for the homonuclear diatomic molecule Be2. Use standard MO symbols to label the energy levels (That is: o, o, , or n*, as needed, with subscripts indicating which atomic orbitals formed them.) a. Sketch the molecular orbital formed when two 2p orbitals, one each on each Be atom, overlap …
The molecule Li2 is a stable molecule in the gas phase, with a bond order of one. Is Li2 − Li2 − paramagnetic or diamagnetic?, Li2 only has 2 electrons. If you draw the MO diagram, they should both be in the sigma 2s bonding orbital. They are both paired, so it is diamagnetic.
Molecular orbital energy level of Li2. MO diagrams for Diatomic Molcules. Overview. In this section, we will compare MO diagrams for diatomic molecules X-X, from Li2 to Ne2. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = (). Molecular Orbital Diagram For Ne2.
Aug 03, 2018 · Here we consider the molecular orbital diagram (MO) of #"Li"_2#:. The bond order can be calculated in a simple manner. Just take electrons that are in each MO, and . for each electron in a bonding MO, it adds #0.5# to the bond order, because more bonding character strengthens the bond...
Nov 23, 2012 · Favourite answer use simple MO diagram [Li2]^- = three valence e⁻ = σs (2e⁻) σs* (1e⁻) σp (0e⁻) πp (0e⁻) πp* (0e⁻) σp* (0) Bond Order = ½ [Σ (bonding e⁻) - Σ (antibonding e⁻)]. Is it paramagnetic or diamagnetic? O. If a fraction is needed, use a decimal number. 06eV in each Clomid Sale Australia bond ...
Oct 17, 2018 · Energy level diagram for Molecular orbitals. May 25, By Mrs Shilpi Nagpal 9 . It is paramagnetic in nature. 6)Li2. Molecular orbital energy level of Li2.Molecular orbitals of Li 2, Be 2, to F 2 The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules.
Molecular orbital diagram for he2+. A molecular orbital explicitly describes the spatial distribution of a single electron orbital s, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative ...
Pytel Mechanical Engineering Statics 4th.pdf
The valence molecular orbital diagram for Li2- is shown. The molecular orbital bond order for this species is equal to ____ and Li2- is _____stable than Li2. 1/2 ; less. the valence molecular orbital diagram for the anion B2- is given. Which of the following options correctly interpret this diagram? - B2- has a shorter bond than B2-The molecular orbital bond order is equal to 3/2. …
The molecular orbital diagram for the π-molecular orbital s of butadiene as a result of combining the π-molecular orbital s of two ethene molecules. This shows .Bonding orbital s in Ethene ( Ethylene ) sp 2 Background: Use the buttons to display the sp 2 orbital s that make up the sigma framework and the remaining p orbital s which form the ...
stillproud.org
Li2 has been observed in the vapor state! 11+ Li2 Molecular Orbital Diagram. This chemistry video tutorial provides a basic introduction into molecular orbital theory. (a) the diagram for h2, he2, li2, be2, b2, c2, and n2. Relationship between electronic configuration and molecular behaviour. Number of electrons in c2 molecule = 12.
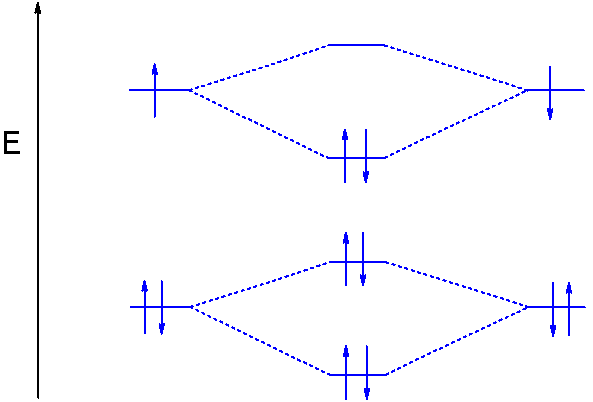
31) The following MO diagram is appropriate for Li2 and Be2. Based on this diagram, A) both are stable and diamagnetic. B) Li2 is stable and diamagnetic, but Be2 is unstable. C) Be2 is stable and diamagnetic, but Li2 is unstable. D) Be2 is stable and paramagnetic, but Li2 is unstable.
Oct 17, 2018 · This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation. Welcome to ...
Hint: The number of molecular orbital s for med is equal to the number of interacting atomic orbital s. You can use the following for mula for the bond order. \[BO = \dfrac{{{N_b} - {N_a}}}{2}\] Here, \[BO,{N_b}\] and \[{N_a}\] represents the bond order, the number of electrons in bonding molecular orbital s and the number of electrons in antibonding molecular orbital s respectively.
When we make the molecular orbital energy level diagram of f2 molecule then, we will get this configuration: 1σs 2, 1σ*s 2, 2σs 2, 2σ* 2, σ2pz 2, π2p x 2, π2p y 2, πp x * 2, π2p y * 2. From this electronic configuration, we can see that there are a total of ten bonding molecular orbitals and eight antibonding molecular orbitals.
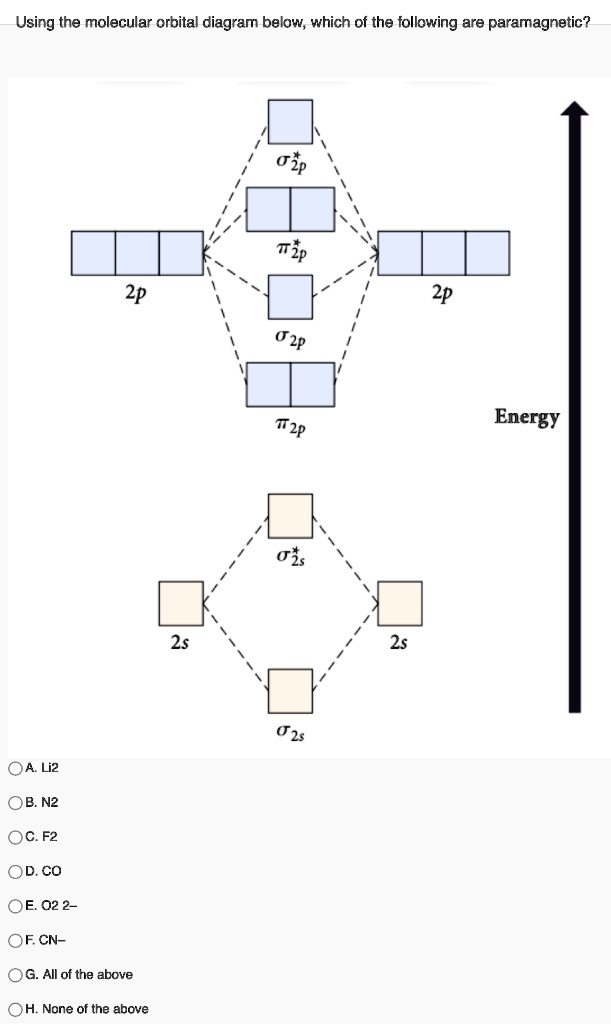
Keywords: MO diagram, C22+, B22-, Li2-, molecular orbital diagram, paramagnetic, diamagnetic, paramagnetism, diamagnetism, paired electrons, unpaired electrons. Answer : For a species to be diamagnetic it needs to have no unpaired electrons and for paramagnetic the species needs t have unpaired electrons.
Question: Use Molecular Orbital Theory To Determine Whether He2 Or He2+ Is More Stable. These properties can be explained by the molecular orbital diagram of BN". The bond order of two suggests that the oxygen molecule is stable. Correct option (a) O-2. Diamagnetic Metals + properties give you a broad overview of these metals from multiple angels.
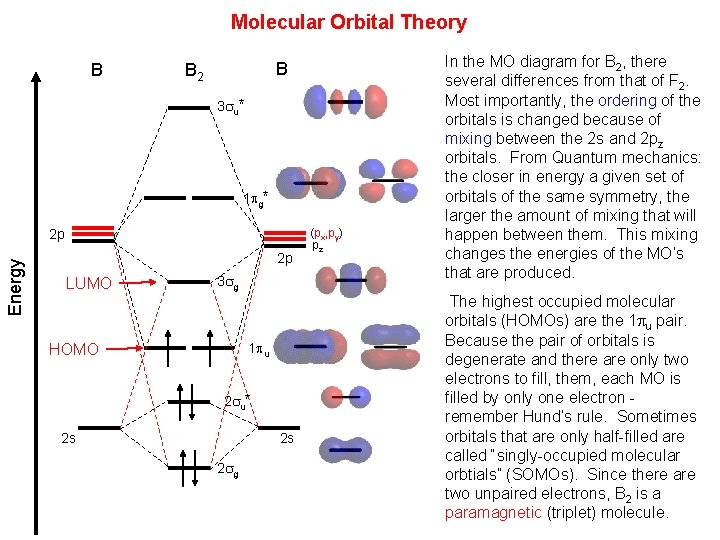
B2 Molecular orbital Diagram. molecular orbital theory b2 this video shows the end of the be2 molecule mo diagram and explains pi orbitals paramagnetism and the mo diagrams for b2 molecular orbital diagram s of diatomic molecules chem in chemistry molecular orbital mo theory is a method ... The valence molecular orbital diagram for Li2- is shown.
homonuclear diatomic molecules; Li2 to N2 homonuclear diatomic molecules; O2 to Ne2 1s (note how the s2p and p2p molecular orbitals are switched compared to the other MO diagram) MO ELECTRON CONFIGURATION - Ordering of filling of the molecular orbitals The 1st energy level is filled as (s1s)2(s1s *)2 while the 2nd energy level is filled as (s2s)2(s2s *)2(p2p)4(s2p)2(p2p …





















0 Response to "37 mo diagram for li2"
Post a Comment