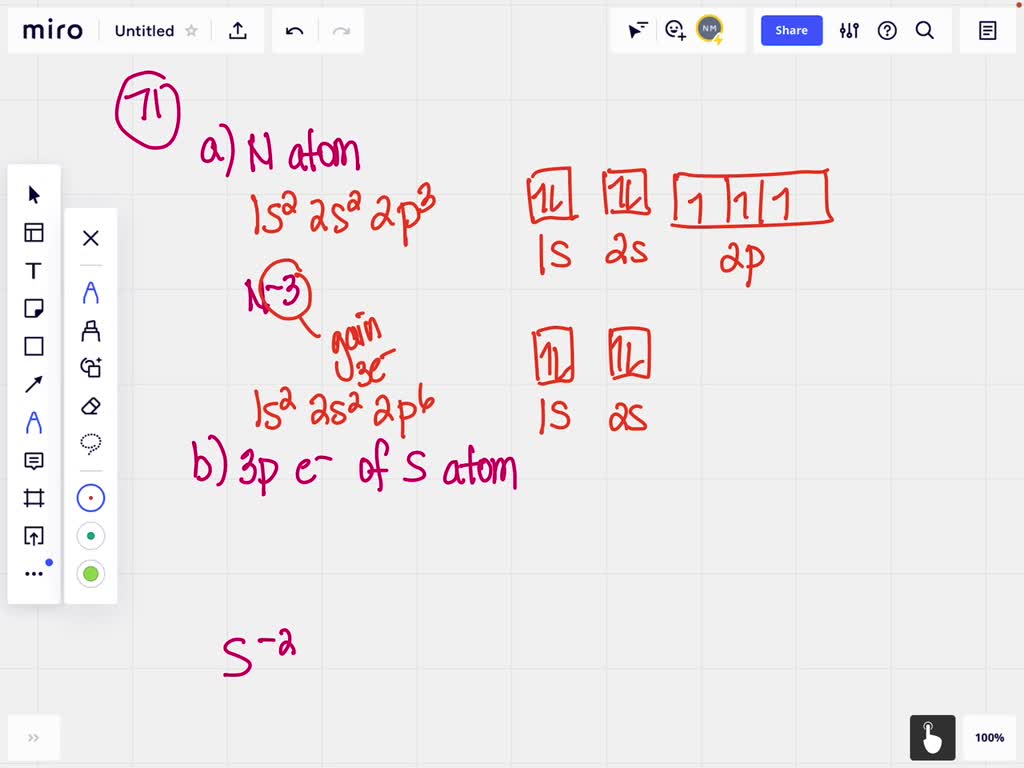
40 show the orbital-filling diagram for n (nitrogen)
Show the orbital filling diagram for br bromine. Orbital notation is a way to show how many electrons are in an orbital for a given element. Notice that the elements k and ca the 4s elements come before the elements sc through zn the 3d elements in the periodic table. Show the orbital filling diagram for bromine. Find orbital diagram near you. The periodic table shows us that nitrogen (N) has an atomic number of 7. As a result, a neutral nitrogen atom will have 7 electrons. In orbital filling diagrams, s-sublevels have 1 orbital and p ...
Draw an orbital diagram for scandium Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for S Status: Resolved. home / study / science / chemistry / chemistry questions and answers ...
Show the orbital-filling diagram for n (nitrogen)
3 Unpaired electrons. Nitrogen atom has total 7 electrons. Two will fill up the n=1 level, and then there are five electrons in the n=2 level. Nitrogen can bond three times with other electrons to fill up it's shell with 8, (8-5=3). And these are those 3 unpaired electrons which were residing the 2p sub-shell of the Nitrogen atom , before the formation of 3 bonds. Show the orbital-filling diagram for (bromine).Status: Resolved. Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top%(15). 1. Describe the two differences between a 2p x orbital and a 3p y orbital. Orbital Filling Diagram for Nitrogen. show the orbital filling diagram for rm n nitrogen best answer the electronic configuration for nitrogen atom is 1s 2 2s 2 2p 3 lowest energy state will have two electrons in s shell which is spherical in shape one spin up and another spin down chemistry problem please help show the orbital filling diagram for nitrogen stack the subshells inorder of energy ...
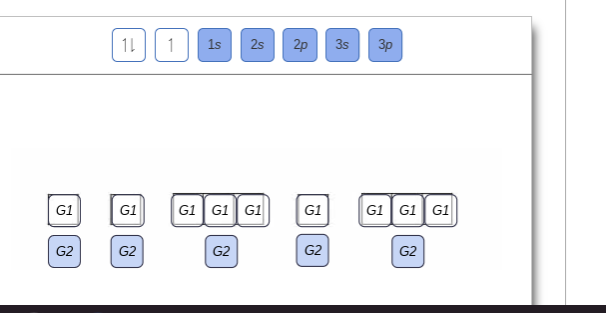
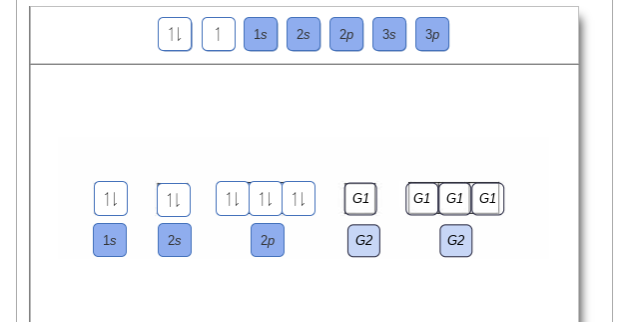
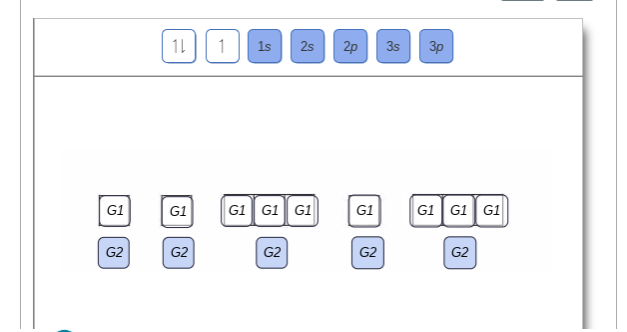
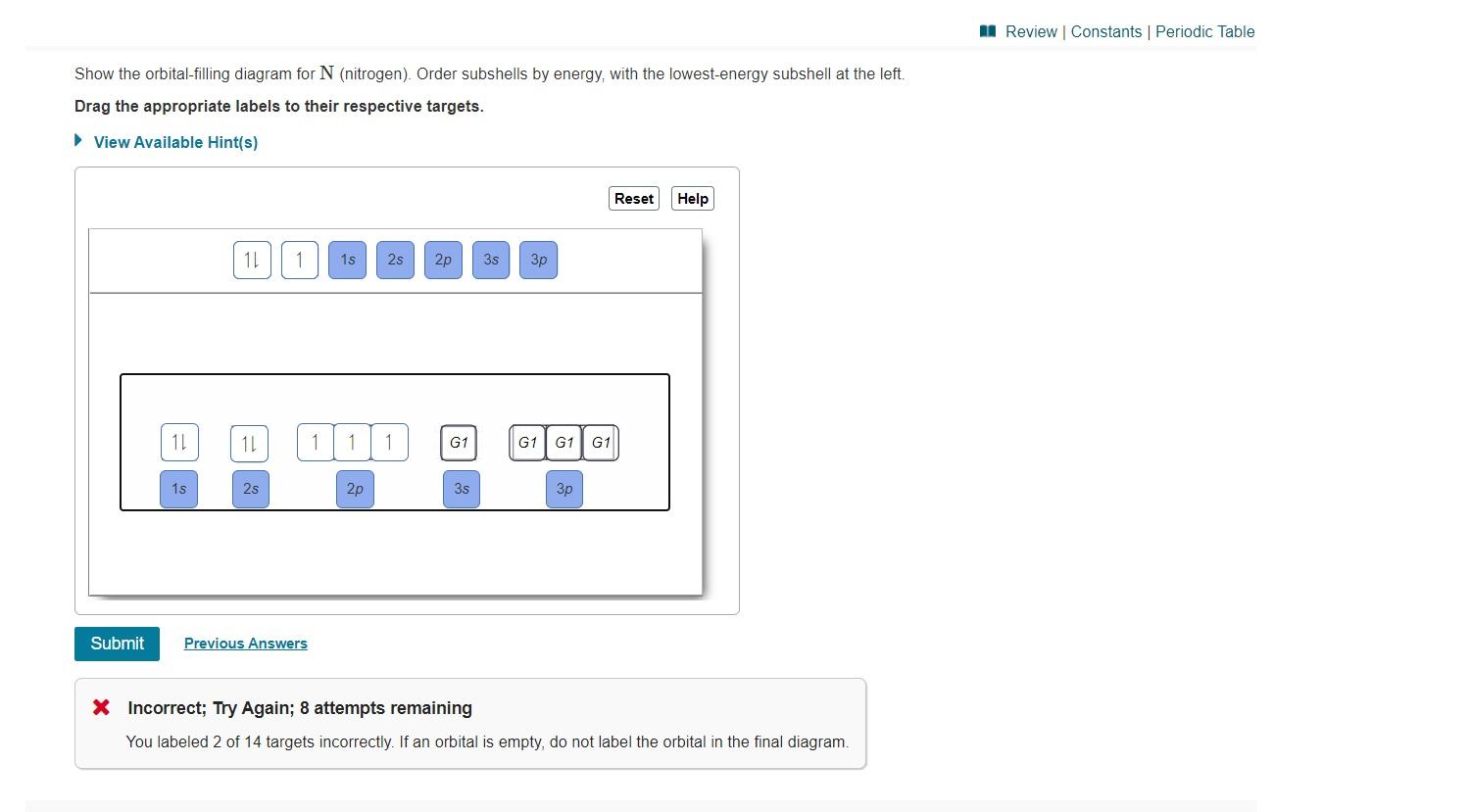
Show the orbital-filling diagram for n (nitrogen). Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint(s) Reset Help 1L 1 1s 2s 2p 3s Зр G1 G1 G1 G1 G1 G1 G1 G1 G1 G2 G2 G2 G2 G2 Part C Show the orbital-filling diagram for S (sulfur). Express your answer numerically as an integer. Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for S (sulfur). Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left Drag the appropriate labels to their respective targets. View Available Hint(s) Reset Help 11 || 1 18 2s 2p 3s ap Group 1 GT G1 G1 G1 GI GT G161 G2 G2 G2 G2 G2 ; Question: Show the orbital-filling diagram for N (nitrogen ... Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Electron ...
Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. 1s²2s²2p³ Orbital-Filling Diagram for Bromine. Bromine has 35 electrons, so it will have 35 arrows placed in its orbital-filling diagram as in figure The order bottom to top . Show the orbital-filling diagram for \rm Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy. Question: Part D Show the orbital-filling diagram for Br (bromine). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint (s) Reset Help 1 18 25 2p 3s 3p 30 48 4p Submit Provide Feedback Next > Part B Show the orbital-filling diagram for N (nitrogen). Since 1s. Use orbital filling diagrams to describe the locations of electrons in an atom. Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p.Show transcribed image text Show the orbital-filling diagram for N. Nitrogen is the seventh element with a total of 7 electrons.
The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Each dot on a circle represents an electron. Periodic Trends. STUDY. PLAY. Item 1: Part A Show the orbital-filling diagram for N (nitrogen). Now that you've mastered the world of electron configurations, it's time to write orbital filling diagrams. This sounds like something that would be tough, but orbital filling diagrams are really just pictures that show you the same thing as electron configurations. Mostly. If you haven't yet learned electron configurations, you really need to go ahead… Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for S (sulfur). In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s. Use orbital filling diagrams to describe the locations of electrons in an atom. Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p .Show transcribed image text Show the orbital-filling diagram for N ...
Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
Show the orbital filling diagram for n nitrogen. Show transcribed image text show the orbital filling diagram for n nitrogen. Use the buttons at the top of the tool to add orbitals. Stack the subshells in order of energy with the lowest energy subshell at the bottom and the highest energy subshell at the top.
Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. To learn to create orbital-filling diagrams. An orbital-filling diagram shows the number of electrons in each orbital, which are shown in order of energy. The placement of electrons in orbitals follows a certain set of ...
The orbital filling diagram of boron. Show the orbital filling diagram for n nitrogen. What Is The Pattern Of Filling In The P Orbitals Socratic Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital filling diagram for boron. Draw an orbital diagram for scandium sc. Orbital filling diagram of boron.
Solved Dra An Orbital Filling Diagram For Valenco Cloctrons And Malch Ihu Correcl Number Unpelrod Oloctrons 0 Ihe Corresponding Atom And Ions Col N Unpaited Oloctrons Cobalt Ill Cation Unpaircd Clectrons A J Unpaircd Clectrons
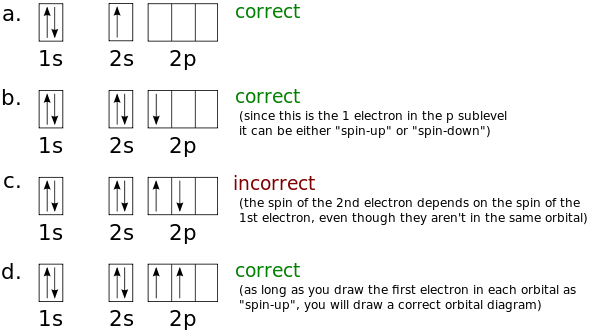
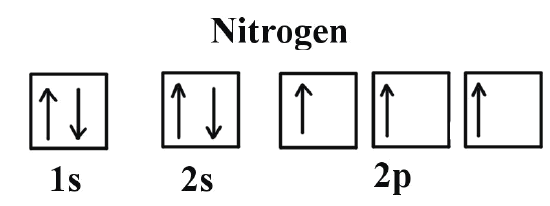
If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital.
Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p . Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be.Given the same amount of absorbed solar energy coming in, the amount of IR escaping to space at the top of the ...
Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital. Therefore the N electron configuration ...
Orbital Filling Diagram for Nitrogen. show the orbital filling diagram for rm n nitrogen best answer the electronic configuration for nitrogen atom is 1s 2 2s 2 2p 3 lowest energy state will have two electrons in s shell which is spherical in shape one spin up and another spin down chemistry problem please help show the orbital filling diagram for nitrogen stack the subshells inorder of energy ...
Show the orbital-filling diagram for (bromine).Status: Resolved. Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top%(15). 1. Describe the two differences between a 2p x orbital and a 3p y orbital.
3 Unpaired electrons. Nitrogen atom has total 7 electrons. Two will fill up the n=1 level, and then there are five electrons in the n=2 level. Nitrogen can bond three times with other electrons to fill up it's shell with 8, (8-5=3). And these are those 3 unpaired electrons which were residing the 2p sub-shell of the Nitrogen atom , before the formation of 3 bonds.
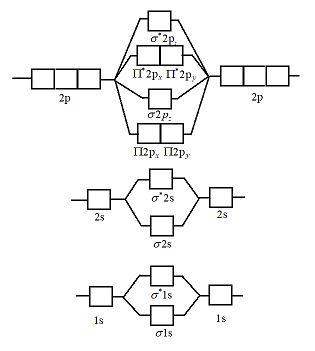
Draw The Molecular Orbital Diagram Of N2 Also Find Its Bond Order And Magnetic Character Chemistry Topperlearning Com 4s4p942zz

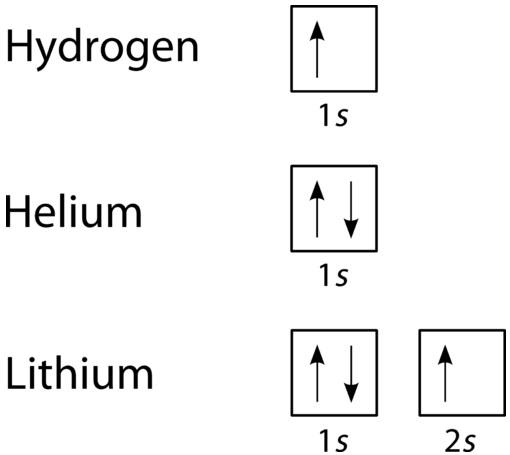
1 Create The Atomic Orbital Of Diagram For Nitrogen 2 Construct The Orbital Diagram For Ni Homeworklib




















0 Response to "40 show the orbital-filling diagram for n (nitrogen)"
Post a Comment