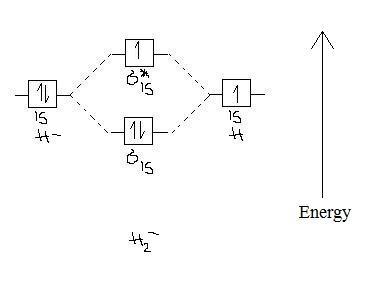
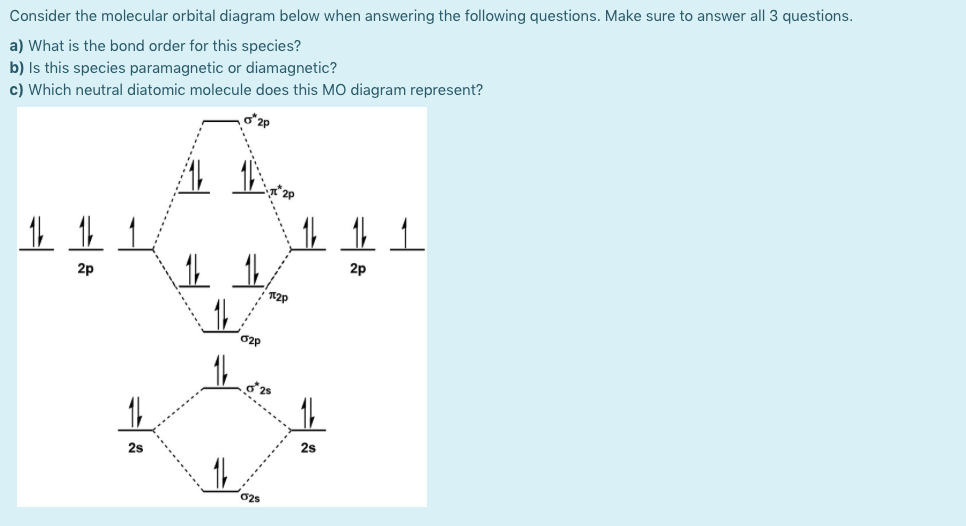
38 use the following mo diagram to find the bond order for o2
On the basis of this principle discuss the conditions for obtaining the maximum yield of SO3 in the following reaction. 2SO2(g)+ O2(g)⇌2SO3(g); ∆𝐻= - 42k.cal.(ii) Calculate the pH value of 0.01M CH3 COOH if it is 5% dissociated. The bond order varies from one molecule to another. Oxygen is a diatomic molecule. Let us first know what is meant by bond order. Bond order. The bond order may be defined as half the difference between the number of electrons in bonding molecular orbitals (Nonbonding) and the number of electrons in the antibonding molecular orbital. Formula ...
Transcribed image text: Use the MO diagram provided below to answer the following questions: • What is the bond order for N? [Select] • Is N2 paramagnetic or diamagnetic?__ (Select] netic? _ [ Select] • What is the bond order for N? (Select] • Is N2 paramagnetic or diamagnetic?__ (Select] • What is the bond order for Not?
Use the following mo diagram to find the bond order for o2
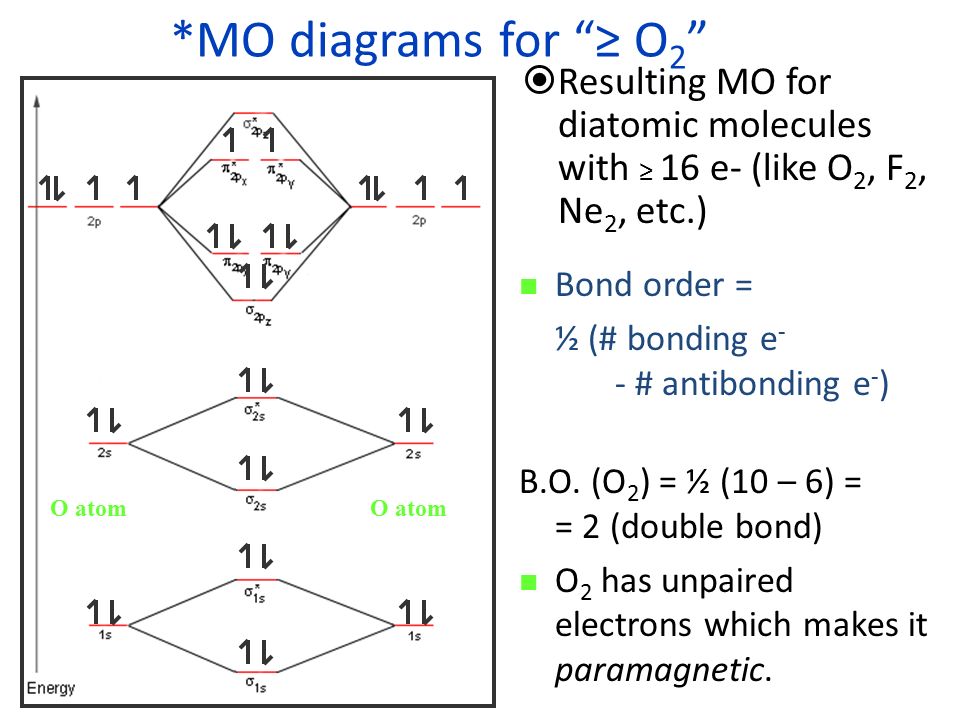
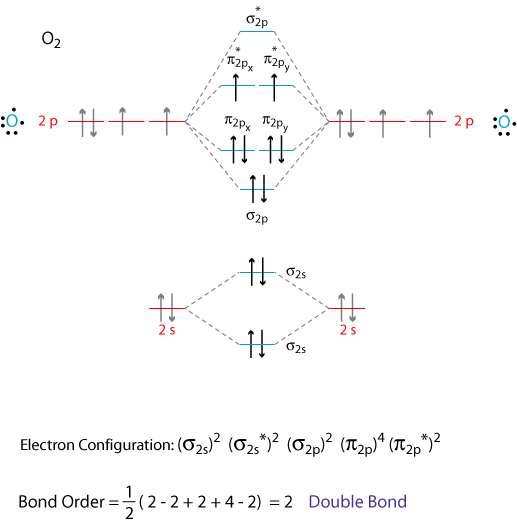
Nov 29, 2021 · Related: Diagram matic; diagram matically. The verb, "to draw or put in the for m of a diagram," is by 1822, from the noun. Related: Diagram med; diagram ming. However, one of the most important molecules we know, the oxygen molecule O2, ... Obtain the molecular orbital diagram for a homonuclear diatomic ion by ... Printable O2 molecular orbital diagrams are available for you to guide your study in the molecular orbital lesson.This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. Electron Configurations and Bond Orders Just as with atoms, we can write a molecular electron configuration for O2 σ2σ*2σ2π4π*2 We can also calculate the O–O bond order: BO 1 2 # bonding e # anti-bonding e 1 2 8 4 2 LCAO MO theory also predicts (correctly) that O2has two unpaired electrons.
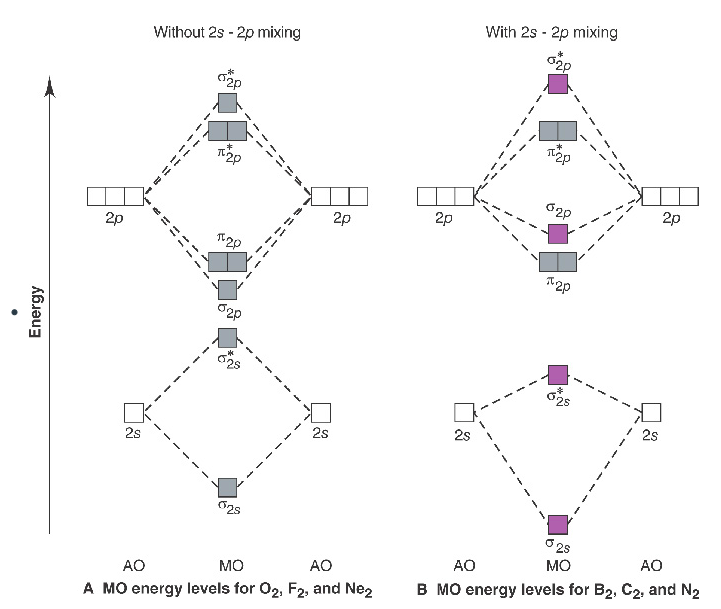
Use the following mo diagram to find the bond order for o2. Solution. Verified by Toppr. As it can be seen from the MOT of O2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [Nb −Na ]/2=[10−6]/2=2. Therefore there is a double bond present as O=O. Transcribed image text: re.com/courses/bb687qu7272es/65878 correct Question 5 0/3.5 pts Use the following MO diagram to find the bond order for O2. Enter a decimal number e.g. 0.5, 1.0, 2.0. Without 2s-2p mixing With 2s-2p mixing CK :O r 25 20 2s AO MO AO AO MO AO A MO energy levels for O Fa. and Ne B MO energy levels for Ba Cz and Ng 0.5 What we see here is a molecular orbital interaction diagram. The middle of the diagram is just the molecular orbital energy diagram. It is analogous to the atomic orbital energy diagram (which goes 1s, 2s, 2p, 3s...). The order of energy so far is σ 1s, σ 1s *. The sides of the diagram just refer back to where those molecular orbitals came from, with dotted lines to guide you from one place to another. 2) Stability of molecules in terms of bond order. Bond order is defined as half of the difference between the number of electrons present in the bonding and antibonding orbitals. Bond Order = ½ ( N b – Na) The molecule is stable if N b > Na ie. bond order is positive. The molecule is unstable if N b < Na i.e. the bond order is negative or zero.
Welcome to Sarthaks eConnect: A unique platform where students can interact with teachers/experts/students to get solutions to their queries. Students (upto class 10+2) preparing for All Government Exams, CBSE Board Exam, ICSE Board Exam, State Board Exam, JEE (Mains+Advance) and NEET can ask questions from any subject and get quick answers by subject teachers/ experts/mentors/students. Electron Configurations and Bond Orders Just as with atoms, we can write a molecular electron configuration for O2 σ2σ*2σ2π4π*2 We can also calculate the O–O bond order: BO 1 2 # bonding e # anti-bonding e 1 2 8 4 2 LCAO MO theory also predicts (correctly) that O2has two unpaired electrons. Printable O2 molecular orbital diagrams are available for you to guide your study in the molecular orbital lesson.This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. Nov 29, 2021 · Related: Diagram matic; diagram matically. The verb, "to draw or put in the for m of a diagram," is by 1822, from the noun. Related: Diagram med; diagram ming. However, one of the most important molecules we know, the oxygen molecule O2, ... Obtain the molecular orbital diagram for a homonuclear diatomic ion by ...























0 Response to "38 use the following mo diagram to find the bond order for o2"
Post a Comment