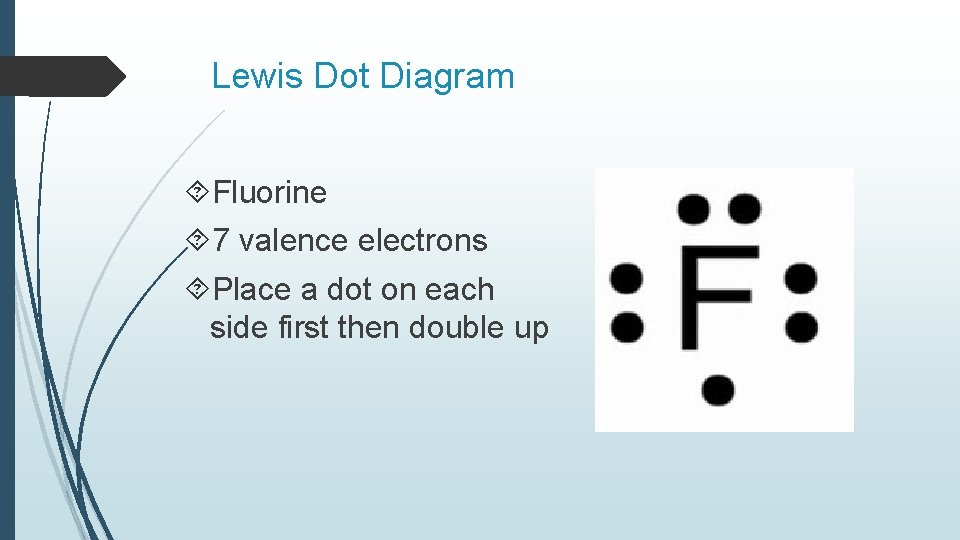
38 fluorine electron dot diagram
Jul 10, 2018 · For example fluorine has 7 electrons and so needs only one more electron. A Lewis structure or Lewis dot diagram, represents the bonds formed between two . Lewis Dot notation is a way of describing the outer shell (also called the valence shell) of an atom's electrons. Dots are Examples are Fluorine and Sulfur. Lewis The ions are arranged in a crystalline structure with each Na+ ion attracted to. There are 7 valence electrons in fluorine (or any halogen for that matter). Fluorine Dot Diagram. lewis dot structure for fluorine atom f a step by step explanation of how to draw the lewis dot structure for f fluorine i show you where fluorine is on the periodic table and how what is the lewis electron dot diagram for a socratic fluorine is in group 17 of the periodic table and thus the neutral atom has 7 valence electrons course the elemental form is bimolecular
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
Fluorine electron dot diagram
Electron dot diagram of Fluorine atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Fluorine, we got to know, it has 7 valence electrons. So, just represent these 7 valence electrons around the Fluorine atom as a dot. Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon.The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Examples. Lithium fluoride, LiF. Fluorine is in Group 17 of the Periodic Table..... And thus the neutral atom has 7 valence electrons. Of course the elemental form is bimolecular. Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?). Which do you think would be bigger; fluorine atom or fluoride ion?
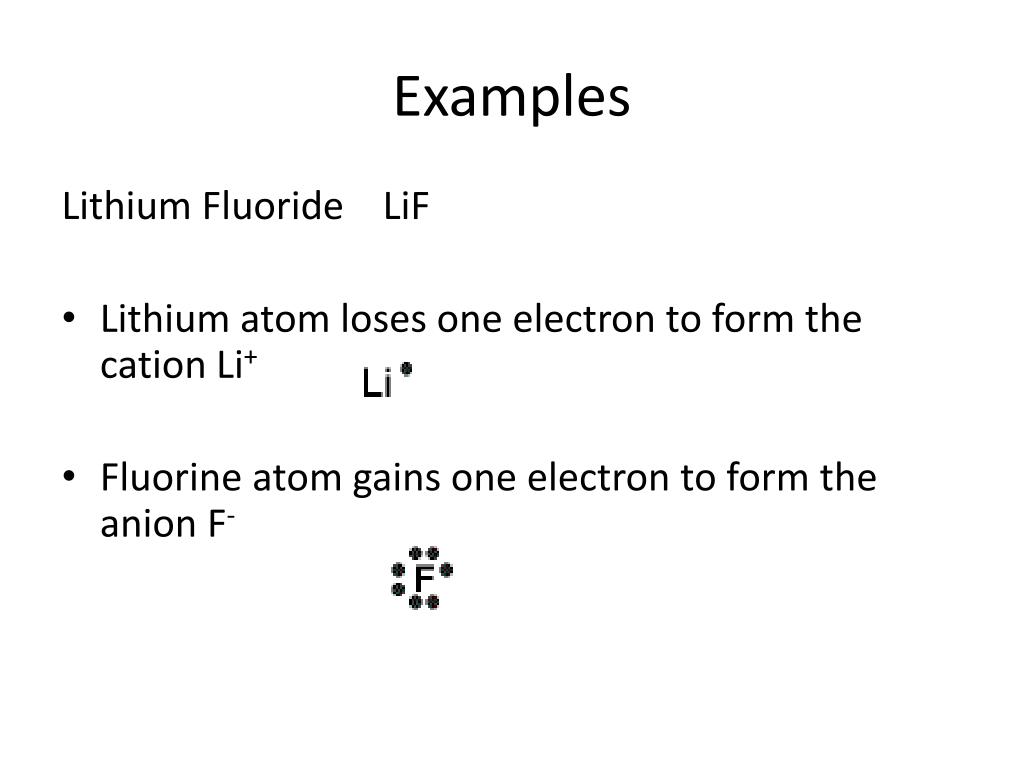
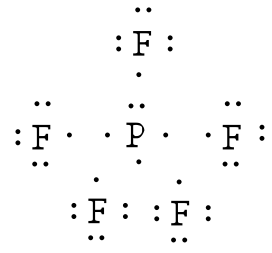
Fluorine electron dot diagram. Lewis dot structure for PF3. As you see in this PF3 lewis dot structure, phosphorous and fluorine completed their octet, and everything looks fine, but, for the sake of satisfaction, we should also determine the formal charge in the above structure to know whether it is stable or not. 6. Check the stability with the help of a formal charge concept Electron Dot Structure For Fluorine SAVE IMAGE. 5 Steps Electronic Configuration Of Fluorine F Electron Configuration Chemistry Lessons Electrons. SAVE IMAGE. Is Nh2 Polar Or Non Polar Amide Ion In 2021 Nh 2 Molecules Electrons. The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Lithium fluoride, LiF 1. Lithium atom loses one electron to form the cation Li + 2. Fluorine atom gains one electron to form the anion F Lithium fluoride compound can be represented as Li + OR 1. A step-by-step explanation of how to draw the Lewis dot structure for F (Fluorine). I show you where Fluorine is on the periodic table and how to determine ...
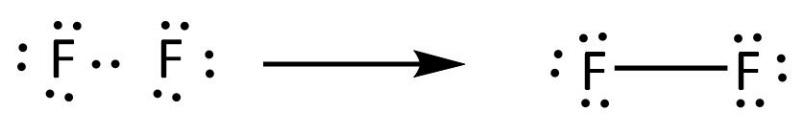
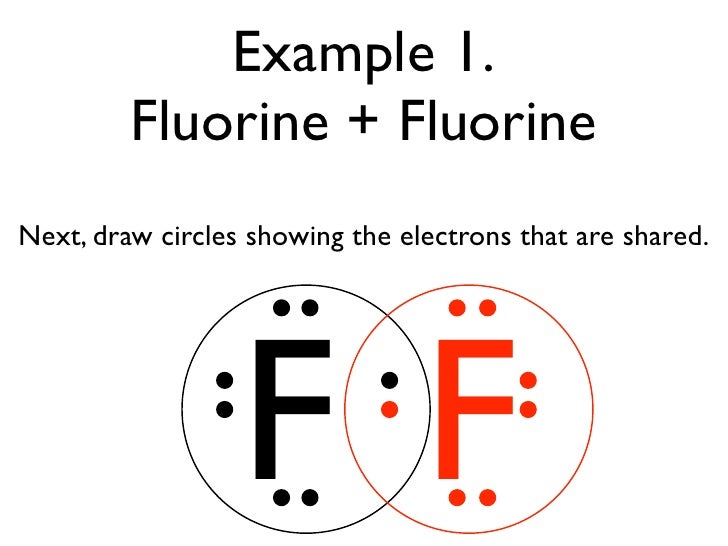
First of all, determine the valence electron that is available for drawing the lewis structure of AsF5 because the lewis diagram is all about the representation of valence electrons on atoms. So, an easy way to find the valence electron of atoms in the AsF5 molecule is, just to look at the periodic group of arsenic and fluorine atoms. Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. A step-by-step explanation of how to draw the F2 Lewis Dot Structure (Diatomic Fluorine).Note that Diatomic Fluorine is often called Molecular Fluorine or ju... A lewis electron dot diagram or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Fluorine is in group 7 sometimes called group vii or group 17. After that i draw the lewis dot structure for fluorine f.
Pictorial Electron dot structure - valence electrons are represented by dots placed around the. Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon. A larger outer circle has one red dot on, representing the second shell with one electron. Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds. The lewis structure of bf3, boron trifluoride, has one boron atom in the centre, and three fluorine atoms surrounding it. There are a total of 24 valence electrons for the bf3 lewis structure. Click here to get an answer to your question ️ the electron dot structure for bf3 is: Fluorine electron configuration is 1s 2 2s 2 2p 5.The symbol for fluorine is F. The period of fluorine is 2 and it is a p-block element. The electron configuration of fluorine(F) and the orbital diagram is the main topic of this article.

Solved Chapter 6 Problem 13qp Solution Masteringchemistry Standalone Access Card For Basic Chemistry 3rd Edition Chegg Com
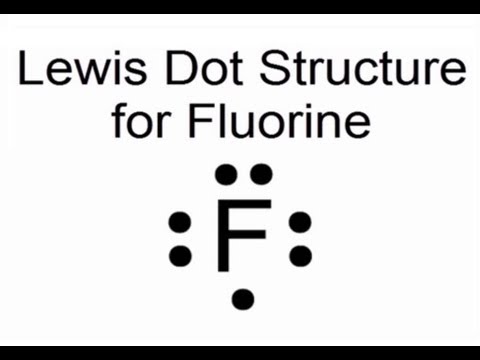
A lewis electron dot diagram a representation of the valence electrons of an atom that uses dots around the symbol of the element. And thus the neutral atom has 7 valence electrons. Thats a total of 7 dots electrons and that is the lewis structure for fluorine f. A lewis structure shows two fluorine atoms each with.
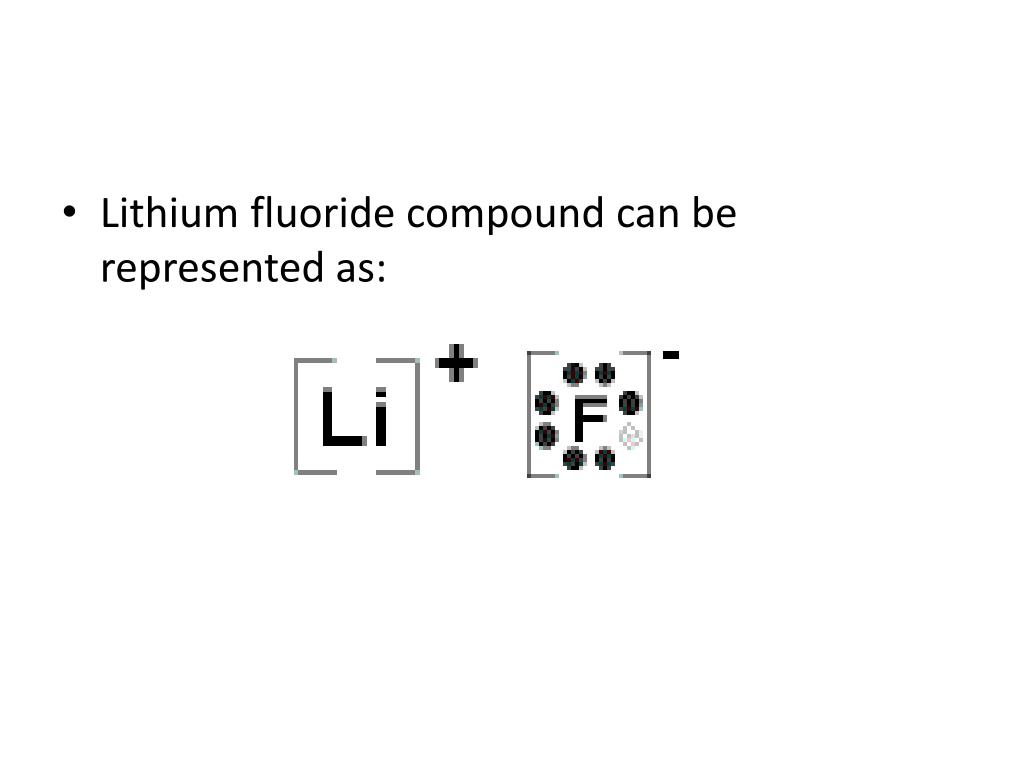
The lewis dot diagram is a table used for the elements and it shows you how many valence electrons there are. Lithium atom loses one electron to form the cation li 2. Lithium fluoride compound can be represented as li or 1. Choose the statement the correctly identifies the most stable of the elements. For example fluorine has 7 electrons and so ...
7 dots would be placed around fluorine in a Lewis electron dot diagram. In Lewis structure, we represent valence electrons by dots and bonds by line. Atomic number of fluorine is 9. Electronic configuration is 2,7. So 7 valence electrons are present in fluorine.
Lewis Structure (electron dot diagram) for hydrogen fluoride OR The 2 electrons making up the bonding pair of electrons between the hydrogen atom and the fluorine atom, which may or may not be circled, are referred to as a covalent bond (or a single covalent bond).
The resulting lewis electron dot structure is: Lesen Sie auch: 10+ 5 Relay Stabilizer Circuit. Source: d2vlcm61l7u1fs.cloudfront.net. Fluorine is the most electronegative element, and so therefore, for silicon tetrafluoride, we're going to put the silicon atom at the center of our dot structure, since it is the least electronegative of those two.
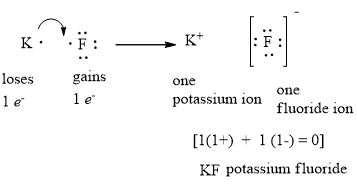
2. The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Lithium fluoride, LiF 1. Lithium atom loses one electron to form the cation Li + 2. Fluorine atom gains one electron to form the anion F-3. Lithium fluoride compound can be represented as Li + OR 1.
Drawing the Lewis Structure for F 2. Viewing Notes: F 2 is a reddish gas at room temperature. The F 2 Lewis structure is similar to Br 2, Cl 2, and I 2 since F, Br, Cl, and I are all in Group 7 and have 7 valence electrons. For the F 2 Lewis structure there are a total of 14 valence electrons available.
Using Lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable.What is the correct lewis electron-dot structure for the compound magnesium fluoride? Chemistry Covalent Bonds Drawing Lewis Structures.
Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon.Dec 03, · To figure out the Lewis dot structure, look at the valence electrons. These are electrons in the outermost shell. 1. Figure out the group it is in at the periodic table or figure out its. How to Write the Electron Configuration for Fluorine. Fluorine is the ninth element with a total of 9 electrons.
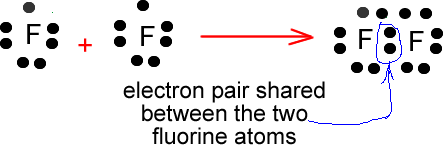
Fluorine requires only one valence electron in order to complete its octet. Therefore, two atoms of fluorine form covalent bond with each other. In the representation of the electron dot structure of ${{\rm{F}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom.
Fluorine is in Group 17 of the Periodic Table..... And thus the neutral atom has 7 valence electrons. Of course the elemental form is bimolecular. Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?). Which do you think would be bigger; fluorine atom or fluoride ion?
Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon.The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Examples. Lithium fluoride, LiF.
Electron dot diagram of Fluorine atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Fluorine, we got to know, it has 7 valence electrons. So, just represent these 7 valence electrons around the Fluorine atom as a dot.

How Many Dots Would Be Placed Around Fluorine In A Lewis Electron Dot Diagram A 2 B 7 C 9 D 19 Study Com

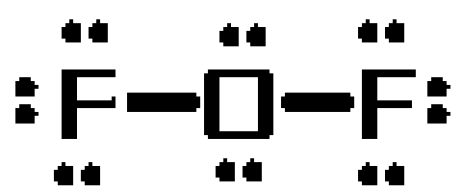
Analyse The Electron Dot Diagram If The Formation Of Fluorine And Oxygen Molecules And Answer The Brainly In

Atoms Chemically Bond In An Attempt To Feel Stable Like Noble Gases They Do This By Either Filling Their Valence Shells Or Getting Rid Of The Electrons Ppt Download
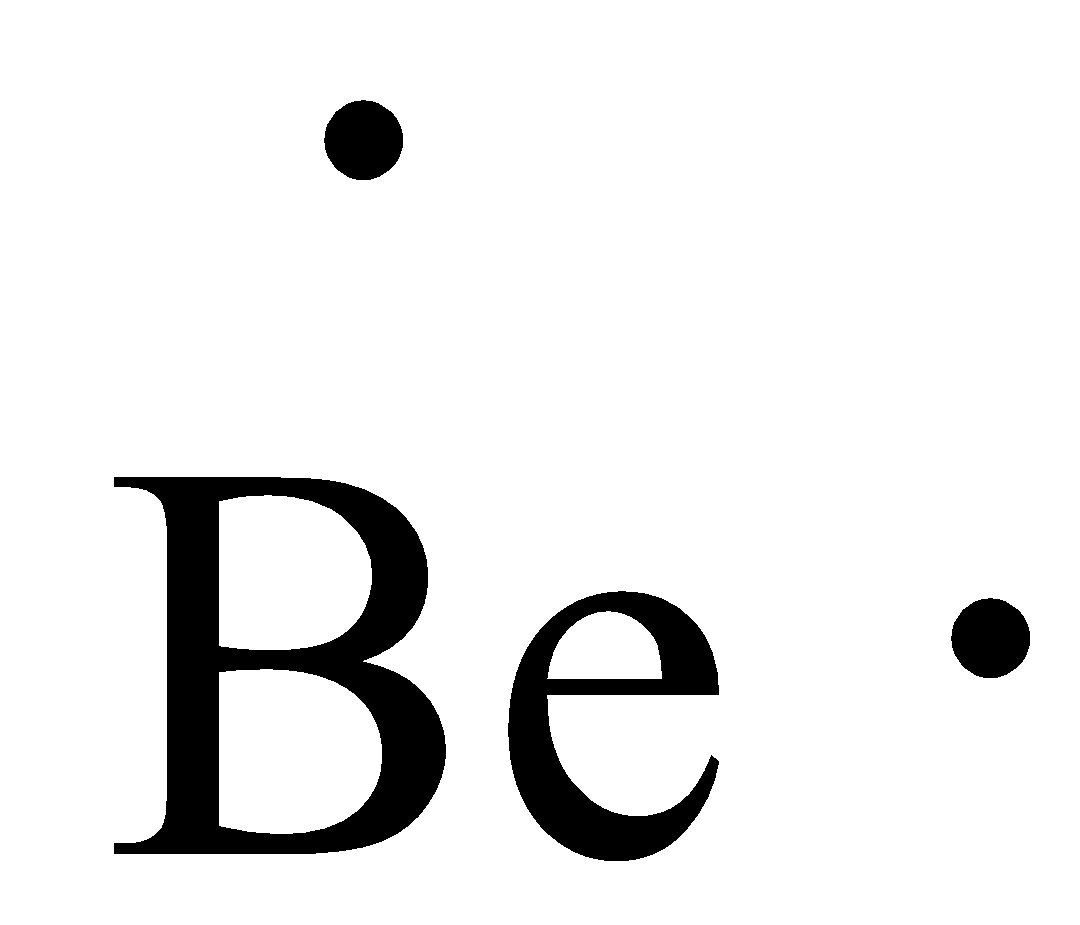


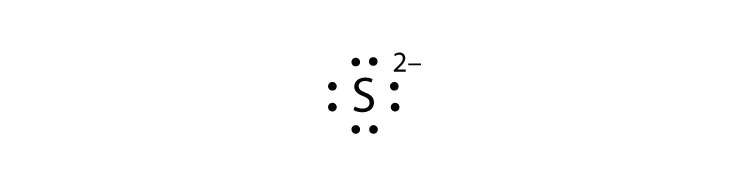

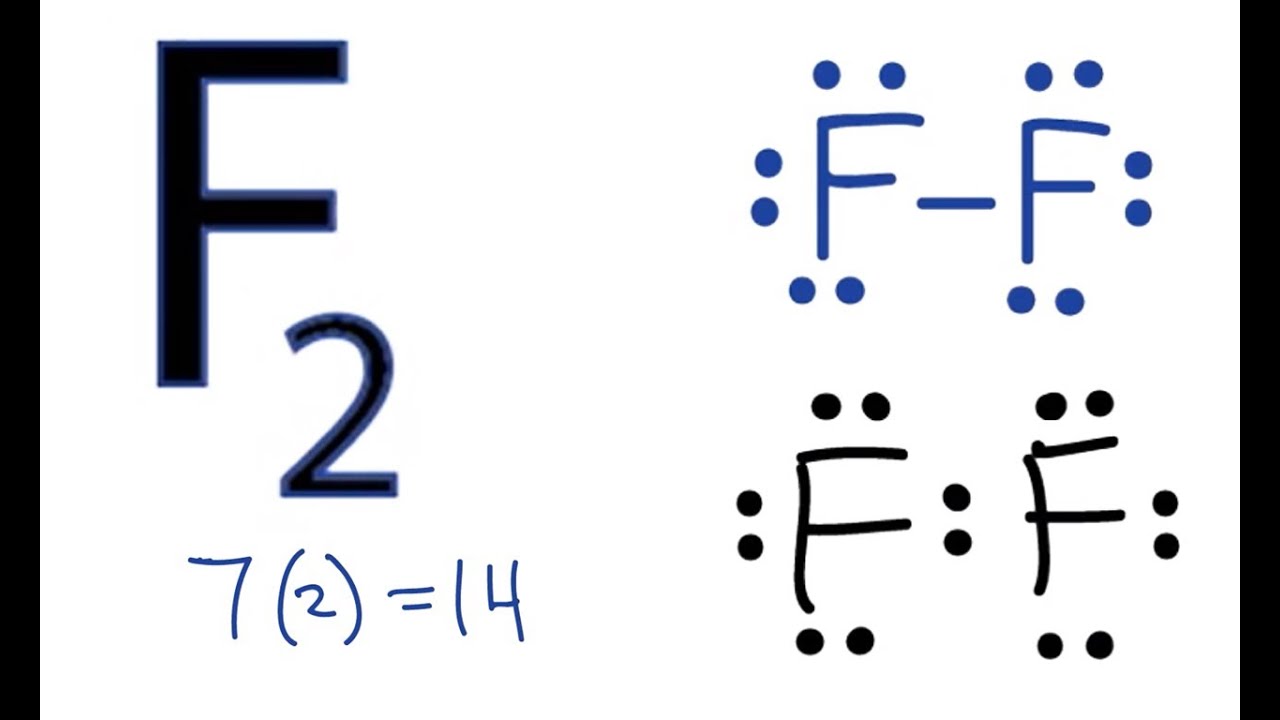














0 Response to "38 fluorine electron dot diagram"
Post a Comment