41 vapor liquid equilibrium diagram
Vapor-Liquid-Liquid Equilibrium (VLLE) - Wolfram ... Aug 04, 2014 · This Demonstration shows phase equilibrium for a binary system of two partially miscible liquids, A and B. Because of the partial miscibility, vapor-liquid equilibrium (VLE), liquid-liquid equilibrium (LLE), and vapor-liquid-liquid equilibrium (VLLE) are present on the phase diagram. You can vary the mole fraction of component B and the heat ... Vapor Pressure - Georgia State University Saturated Vapor Pressure The process of evaporation in a closed container will proceed until there are as many molecules returning to the liquid as there are escaping. At this point the vapor is said to be saturated, and the pressure of that vapor (usually expressed in mmHg) is called the saturated vapor pressure.
VAPOR- LIQUID EQUILIBRIUM DIAGRAM IN ASPEN PLUS - YouTube In this video we will learn about fundamentals of vapor-liquid equilibrium and plotting the diagram in Aspen Plus.Email ID: cheme.friends@gmail.comInstagram:...

Vapor liquid equilibrium diagram
vlle - LearnChemE Vapor-Liquid-Liquid-Equilibrium Description This is a simulation of phase equilibrium for one mole of a binary system (A, B) that forms two partially-miscible liquids (α, β). The T-x-y diagram shows vapor-liquid equilibrium (VLE), liquid-liquid equilibrium (LLE), and vapor-liquid-liquid equilibrium (VLLE). Vapor Liquid Equilibrium - Bucknell University Vapor-liquid equilibrium (known as VLE) is the state in which the rate of condensation is equal to the rate of evaporation so there is no net vapor-liquid interconversion ( Wikipedia ). Typically, a substance in vapor-liquid equilibrium is at its boiling point. A boiling point diagram for a binary mixture is found to the right. Vapor - Wikipedia Vapor refers to a gas phase at a temperature where the same substance can also exist in the liquid or solid state, below the critical temperature of the substance. (For example, water has a critical temperature of 374 °C (647 K), which is the highest temperature at which liquid water can exist.) If the vapor is in contact with a liquid or solid phase, the two phases will be in a state of ...
Vapor liquid equilibrium diagram. VAPOR-LIQUID EQUILIBRIUM - Thermopedia VAPOR-LIQUID EQUILIBRIUM ... This equation gives the relationship between the saturation vapor pressure and specific (molar) thermodynamic properties of the ... VLE phase diagram, residue curve map - VLE - Calc .com Calculation of vapor-liquid equilibrium (VLE) and drawing of phase diagrams. Name of substance. CAS-nr. Formula. Type of substance. acetone. 67-64-1. C 3 H 6 O. ketone. Vapor-Liquid Equilibrium Diagram for Non-Ideal Mixture ... Download Wolfram Player. This Demonstration shows and diagrams for vapor-liquid equilibrium (VLE) of a benzene/ethanol mixture. This liquid mixture is non-ideal, and the system has an azeotrope (a condition where liquid and vapor have the same composition). The blue curve represents the liquid-phase boundary (bubble point), and the green curve ... VLE-diagrams, distillation calculations Main applications of vapor-liquid equilibrium calculations Pure compound properties Boiling point, heat of vaporization, heat capacity, vapor pressure, critical data, e.t.c. * NEW SUBSTANCES * amines, heterocycles, gases (supercritical components!) VLE -diagram Vapor pressure or boiling temperature in function of mixture composition.
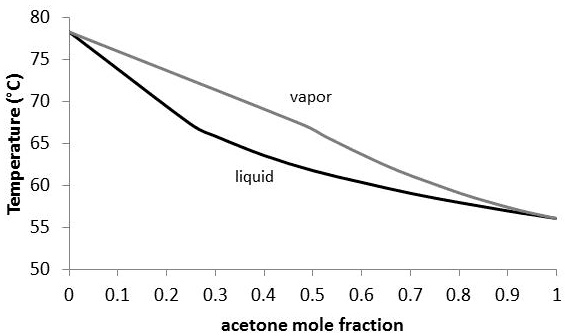
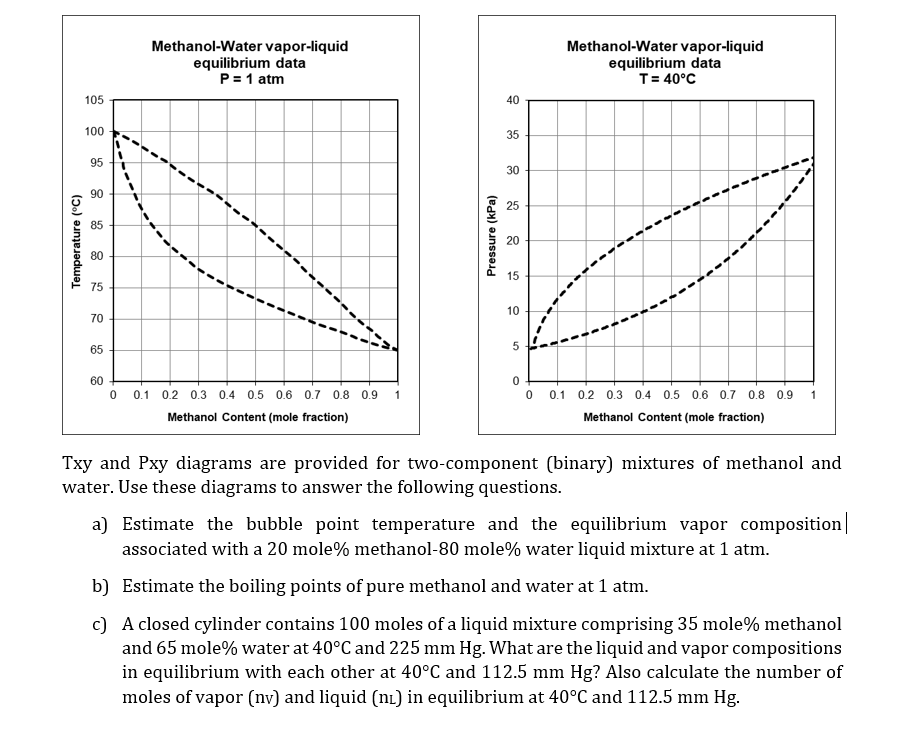
PDF Fundamentals of Vapor-liquid Phase Equilibrium (Vle) 1.2 BINARY VLE PHASE DIAGRAMS Two types of vapor-liquid equilibrium diagrams are widely used to represent data for two-component (binary) systems. The first is a "temperature versus x and y" diagram (Txy). The x term represents the liquid composition, usually expressed in terms of mole fraction. The y term represents the vapor composition. Vapor-Liquid Equilibrium - an overview | ScienceDirect Topics A vapor-liquid equilibrium (VLE) graph is plotted using the ICAS program to find out the composition of azeotrope. The solute with less concentration at azeotrope is tentatively selected i.e. ethanol is the target solute in the ethanol + hexane azeotrope since the amount of ethanol is ~ 21 % mol at azeotrope. PDF Chapter 10 Vapor/Liquid Equilibrium - kau Vapor/Liquid Equilibrium • Previous chapters dealt with pure substances or with constant composition mixtures (air). • But in chemical reactions and number of industrially mass-transfer operations the . composition changes are the desired outcome. (Process such as distillation, absorption and extraction bring phases of different vapor-liquid-equilibrium-diagram-for-non-ideal-mixtures ... Vapor-Liquid Equilibrium Diagram for Non-Ideal Mixtures. This simulation shows P-x-y and T-x-y diagrams for vapor-liquid equilibrium (VLE) of a benzene/ethanol mixture. This liquid mixture is non-ideal, and the system has an azeotrope (a condition where liquid and vapor have the same composition). The blue curve represents the liquid-phase ...
PDF Lecture 7. Vapor-Liquid Equilibria - Weebly 7 Vapor-Liquid Equilibria and Saturation Prof. Manolito E Bambase Jr. Department of Chemical Engineering. University of the Philippines Los Baños SLIDE 25 Vapor-Liquid Equilibrium for Multi-component Systems Consider a binary mixture with components A and B. Liquid X A0 X B0 Vapor Y A (P A) Y B (P B) Liquid X A X B Heating Vapor-liquid_equilibrium - chemeurope.com vapor-liquid equilibrium, abbreviated as vle by some, is a condition where a liquid and its vapor (gas phase) are in equilibrium with each other, a condition or state where the rate of evaporation (liquid changing to vapor) equals the rate of condensation (vapor changing to liquid) on a molecular level such that there is no net (overall) … PDF Fundamentals of Vapor-liquid Equilibrium (Vle) Figure 1.4 Txy diagrams at two pressures. 4FUNDAMENTALS OF VAPOR-LIQUID EQUILIBRIUM (VLE) when the Go button is clicked to generate the Txy diagram. As shown in Figure 1.5 , this windowalsogivesatable ofdetailed information.ThewindowshowninFigure 1.6opens, and xy picture is selected. Drawing Vapor-Liquid Equilibrium Diagrams - Student ... Posted 14 February 2010 - 10:04 PM I am asked to calculate and draw the vapor-liquid equilibrium (VLE) diagram of a binary mixture of acetone and glycerol, given only the total pressure, P = 1 atm (760 mmHg). It says for me to explicitly plot the mole fraction of acetone versus the mole fraction of glycerol. I have no idea where to start.
Vapor-liquid equilibrium enthalpy-composition diagrams ... The equilibrium vapor and liquid compositions may be obtained from Y-X or Y-X diagrams. Saturated vapor and liquid points on the H-X diagram at equilibrium with each other are joined by straight lines called tie lines. The single-stage graphical representation described in Section 5.3.1 is an illustration of a tie line.
Vapor–liquid equilibrium - Wikipedia Vapor-liquid equilibrium diagrams Vapor-Liquid Equilibrium Diagram For each component in a binary mixture, one could make a vapor-liquid equilibrium diagram. Such a diagram would graph liquid mole fraction on a horizontal axis and vapor mole fraction on a vertical axis.
Vapor Liquid Equilibria for Distillation Columns - COSTELLO Vapour-Liquid-Equilibrium (VLE) Curves Constant pressure VLE data is obtained from boiling point diagrams. VLE data of binary mixtures is often presented as a plot, as shown in the figure on the right. The VLE plot expresses the bubble-point and the dew-point of a binary mixture at constant pressure.
Vapor-Liquid Equilibrium - an overview | ScienceDirect Topics The phase diagram depends upon the Gibbs energies of vaporization of the pure components, Δ gv (Zn)o = ( μZnv − μZno (l)) and Δ gv (Mg)o = ( μMgv − μMgo (l)) as shown in Fig. 5.3. Sign in to download full-size image Fig. 5.3. Pressure-composition phase diagram of the Zn-Mg system at 977°C calculated for ideal vapor and liquid solutions.
10.11: Vapor-Liquid Equilibrium - Chemistry LibreTexts The magnitude of the vapor pressure of a liquid depends mainly on two factors: the strength of the forces holding the molecules together and the temperature. It is easy to see that if the intermolecular forces are weak, the vapor pressure will be high. Weak intermolecular forces will permit molecules to escape relatively easily from the liquid.
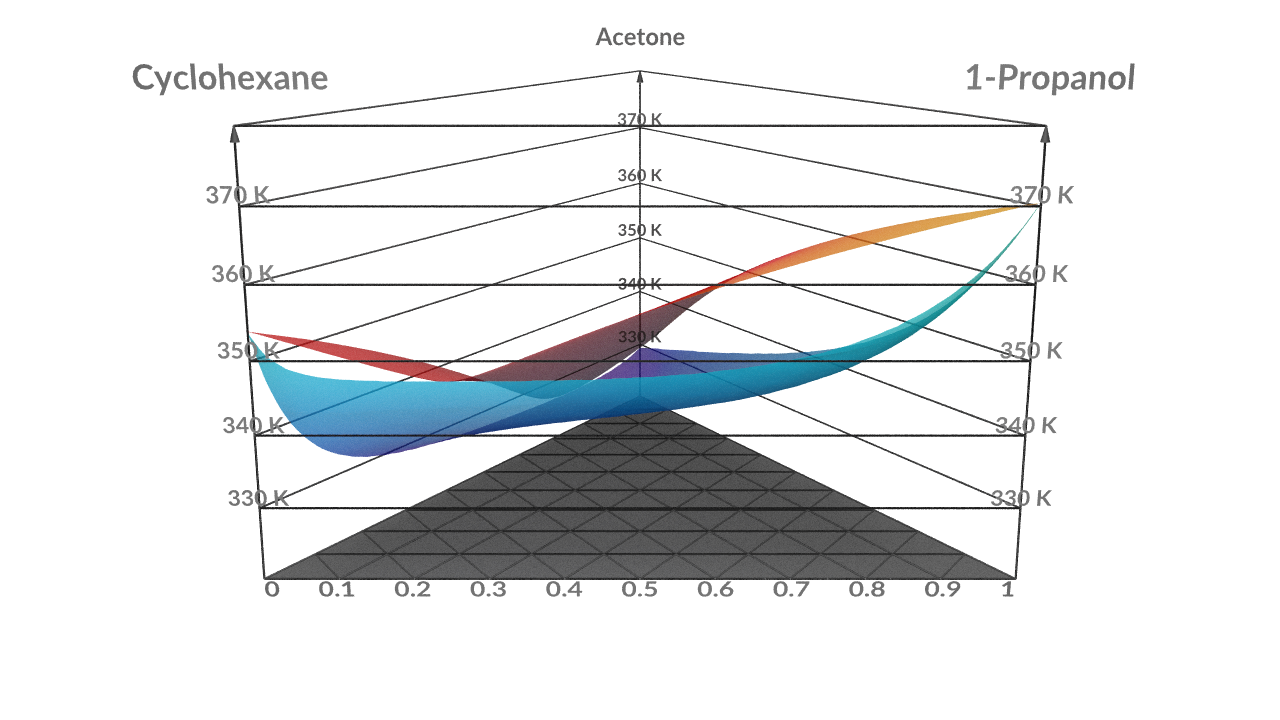
Ternary Phase Diagram - an overview | ScienceDirect Topics A ternary phase diagram shows possible phases and their equilibrium according to the composition of a mixture of three components at constant temperature and pressure. Figure 4.23 shows a schematic of a ternary phase diagram. Single-phase regions are areas that originate from the vertex of the triangle and that are not enclosed by black curves. Two-phase regions …
DOC Vapor Liquid Equilibrium diagrams of binary systems - Elsevier Vapor Liquid Equilibrium diagrams of binary systems (Continued) Figure M-55 Methanol - Benzene Figure M-81 Two propanol - Water Figure M-56 Methanol - Ethyl acetate Figure M-82 Water - 1 Butanol Figure M-57 Methanol - Isopropyl alcohol Figure M-83 Water - Acetic acid Figure M-58 Methanol - Water Figure M-84 Water - Formic acid ...
Vapor-Liquid Equilibrium of Non-Ideal Solutions. Vapor-liquid equilibrium data for Ethanol-Water* n-Buianol-Wator# Ethyl Aeeiate-Water# and n-Hexane-Ethanol at atmospheric pr 00 sura and £or n-Hoxana-Sthanol at 2f50 mnu, 59$ mm.* 1270 mm*# Ijbj mm** 2J10 mm.#
Isobaric Vapor–Liquid Phase Diagrams for ... by N Shardt · 2018 · Cited by 15 — Figure 1. Schematic diagrams of vapor (V)–liquid (L) systems contained in a reservoir that has pressure PR and temperature TR ...
2. (30 pts) Vapor Liquid equilibrium diagram: Prepare ... Chemical Engineering questions and answers. 2. (30 pts) Vapor Liquid equilibrium diagram: Prepare a complete T-xy diagram (constant pressure 1 atm) for the binary system acetone and methyl ethyl ketone. Perform your calculations (fill in the blank table below with your calculated results) and then show your answer on the blank graph given below.
Vapor-liquid Equilibrium Diagrams | Matlab/Scientific ... Vapor-liquid Equilibrium Diagrams. Uses Simulink to compute Vapor-Liquid Equilibrium diagrams for ethanol-water binary mixture. To compute activity coefficients, program uses Van-Laar Model. This mixture presents a positive azeotrope. Requirements: · MATLAB Release: R12 · Simulink
Ternary Phase Diagrams The liquid path then leaves the boundary curve at U and moves across the field where pure D is crystallizing. When the liquid composition reaches point N on the boundary curve between D + LIQ and C + LIQ, crystals of C begin to form. The liquid then moves along the boundary curve toward the eutectic, E.
Distillation - Wikipedia Equilibrium stages are ideal steps where compositions achieve vapor–liquid equilibrium, repeating the separation process and allowing better separation given a reflux ratio. A column with a high reflux ratio may have fewer stages, but it refluxes a large amount of liquid, giving a wide column with a large holdup.
VLE VAPOR LIQUID EQUILIBRIUM - Introduction SIMPLE MODELS FOR VAPOR/LIQUID EQUILIBRIUM 3.1 Raoult's Law • Assumptions: - The vapor phase is an ideal gas (low to moderate pressure) - The liquid phase is an ideal solution (the system are chemically similar) *Chemically similar: the molecular species are not too different in size and are of the same chemical nature. eg: n-hexane/n ...
PDF Chapter 10 Vapor/Liquid Equilibrium: Introduction A schematic three-dimensional diagram illustrating these surfaces for VLE is shown in Fig. 10.1. This figure shows schematically the P-T-composition surfaces which contain the equilibrium states of saturated vapor and saturated liquid for a binary system.
Liquid-Vapor Equilibrium of a Binary System | Chem Lab Liquid-Vapor Equilibrium of a Binary System. Adapted by J. M. McCormick from an exercise used at the University of Kansas. Last Update: December 19, 2012. Introduction. The understanding of the equilibrium between the liquid and vapor phases in a multi-component system is important industries ranging from brewing to petroleum refining.
Lab Report 3 liquid vapor phase diagram - CHEM111 ... A liquid-vapor phase diagram plots temperature of mixture versus the mole fraction or composition. A phase diagram of 2 components that will have differing molecular interactions causing mixture's boiling point to rise/drop will show an azeotrope, in which there will be 2 lobes that either concave down/up depending on if there's positive ...
Vapor - Wikipedia Vapor refers to a gas phase at a temperature where the same substance can also exist in the liquid or solid state, below the critical temperature of the substance. (For example, water has a critical temperature of 374 °C (647 K), which is the highest temperature at which liquid water can exist.) If the vapor is in contact with a liquid or solid phase, the two phases will be in a state of ...
Vapor Liquid Equilibrium - Bucknell University Vapor-liquid equilibrium (known as VLE) is the state in which the rate of condensation is equal to the rate of evaporation so there is no net vapor-liquid interconversion ( Wikipedia ). Typically, a substance in vapor-liquid equilibrium is at its boiling point. A boiling point diagram for a binary mixture is found to the right.
vlle - LearnChemE Vapor-Liquid-Liquid-Equilibrium Description This is a simulation of phase equilibrium for one mole of a binary system (A, B) that forms two partially-miscible liquids (α, β). The T-x-y diagram shows vapor-liquid equilibrium (VLE), liquid-liquid equilibrium (LLE), and vapor-liquid-liquid equilibrium (VLLE).









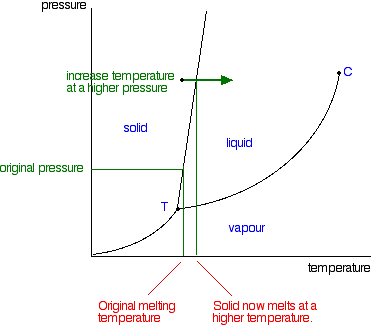

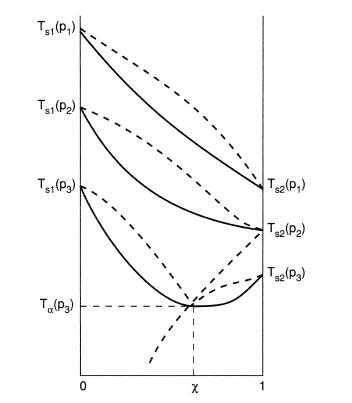



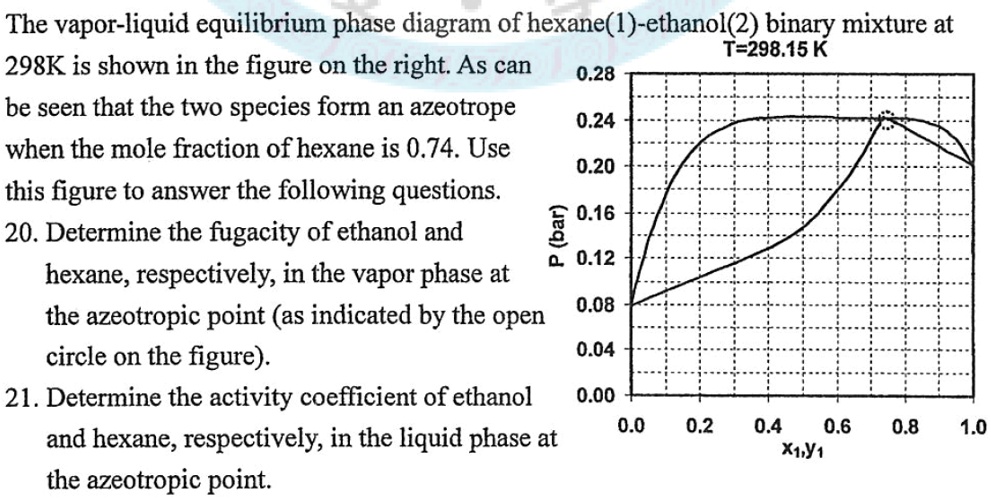
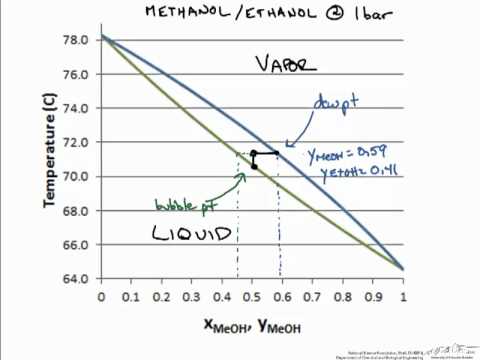




0 Response to "41 vapor liquid equilibrium diagram"
Post a Comment