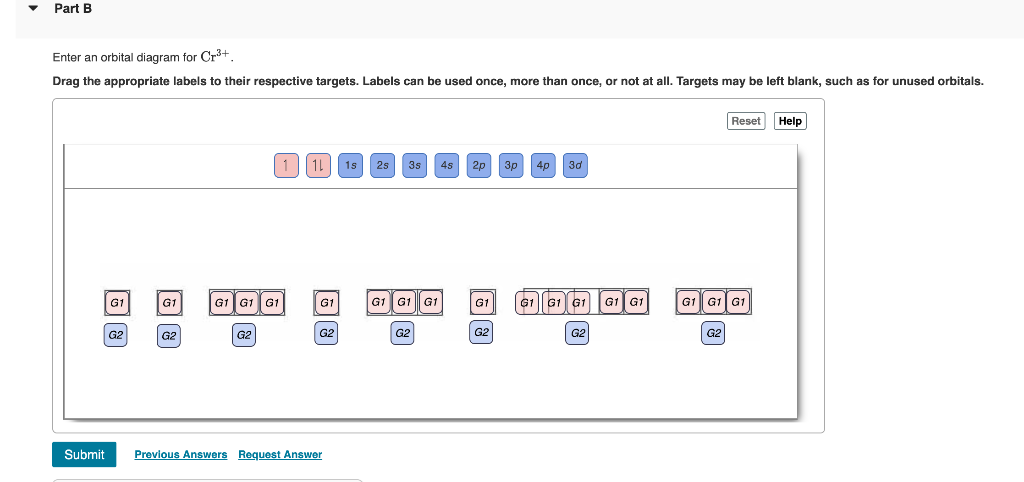
39 orbital diagram for cr3+
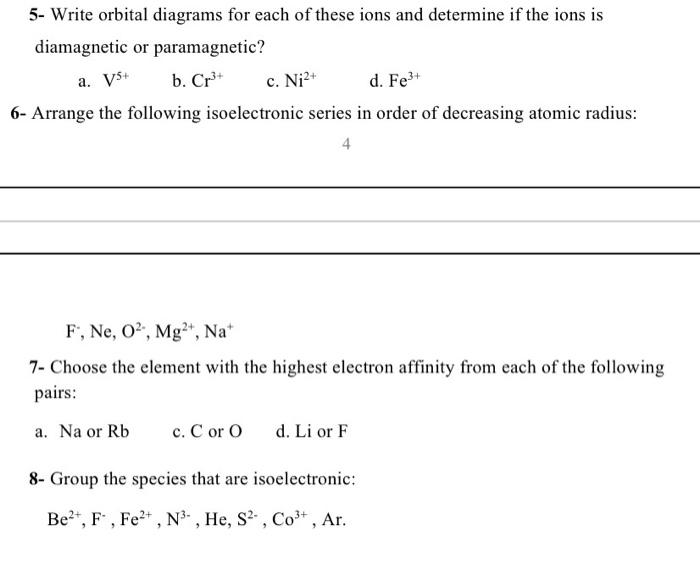
Solved Part A Enter an orbital diagram for V5+ Drag the ... Transcribed image text: Part A Enter an orbital diagram for V5+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all targets will be filled Reset Help P 111 1s 25 38 4s 2p 3p 4p 3d 61 G1 G1 GIG1 G1 GIG1 G1 G1 G1G1G1 G1|| G1 G16161 62 G2 G2 G2 G2 G2 G2 G2 Submit Request Answer Part B Enter an orbital diagram for Cr3 Drag the ... Orbital Diagram For V5+ - schematron.org Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V. Since the 4s orbital is higher in energy, its electrons will be removed first. Not that it matters here, though, because exactly 5 electrons are.
How to Write the Atomic Orbital Diagram for Chromium (Cr ... To write the orbital diagram for the Chromium (Cr) first we need to write the electron configuration for just Cr. To do that we need to find the number of e...

Orbital diagram for cr3+
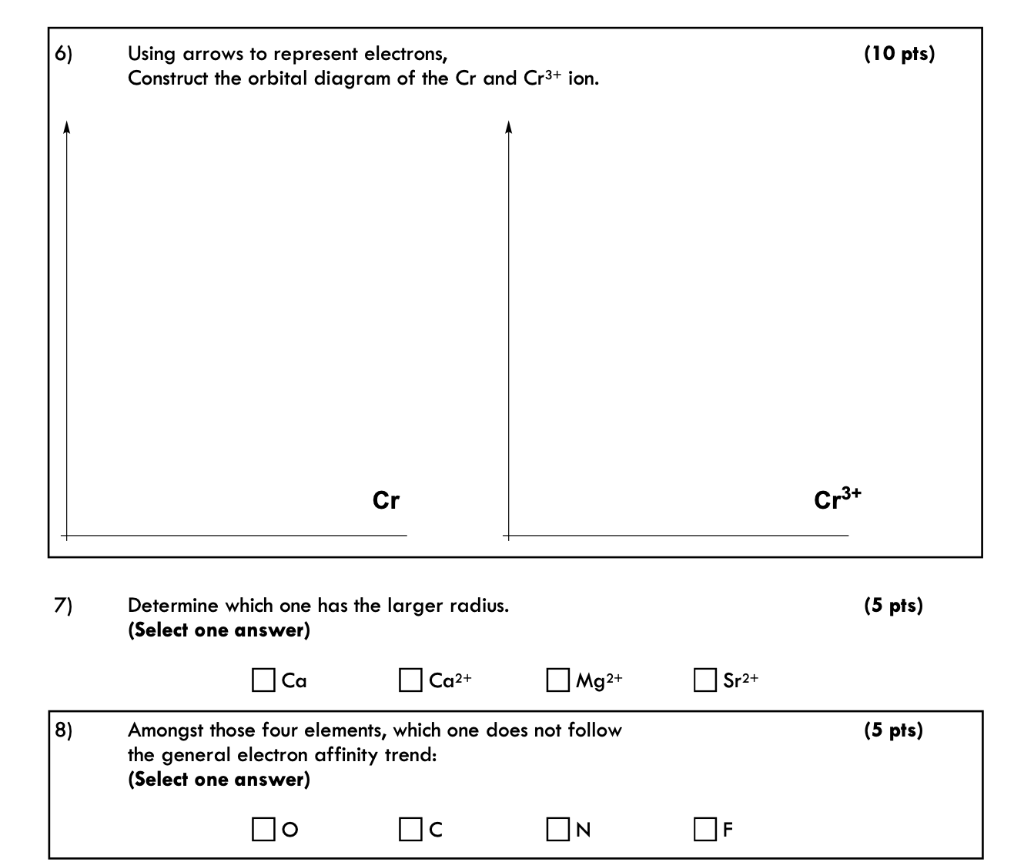
PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? What is the orbital diagram for chromium? - Quora commanly d-subshell have 5-d orbitals. each one orbital contain 2 electrons. so, totally 10 electrons. Half filled d5 & full filled d10 orbitals are more stable so, E.C of cr (24) is writen as [Ar]3d5 4s1 instead of [Ar]3d4 4s2 because half filled E.C is more stable. 5.8K views View upvotes Related Answer Aryan Jha Answered: 6) Using arrows to represent electrons,… | bartleby 6) Using arrows to represent electrons, Construct the orbital diagram of the Cr and Cr3+ ion. Cr Cr3+ Determine which one has the larger radius. (Select one answer) 7) O Ca Ca2+ OMg2+ Sr2+ 8) Amongst those four elements, which one does not follow the general electron affinity trend: (Select one answer) ON OF ఐ Question Need solution urgently
Orbital diagram for cr3+. Orbital Diagram For Fe3+ Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms. Orbital Diagram For Strontium - schematron.org In an orbital (box) diagram a box represents each notation and an orbital diagram. strontium atom (a) in the spdf notation and (b) in the. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of strontium (atomic number: 38), the most. Solved Part B Enter an orbital diagram for Cr3+. Drag the ... Part B Enter an orbital diagram for Cr3+. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Targets may be left blank, such as for unused orbitals. V5 Orbital Diagram Solution for Write an orbital diagram for all subshells beyond the noble gas core for each of the following. a. V5+ b. Orbital Diagram For V5+ Cr2+ c. Zr2+ d. Au+. Write orbital diagrams for each of these ions. V5+ Cr3+ Ni2+ Fe3+ Determine if the ion is diamagnetic or paramagnetic.
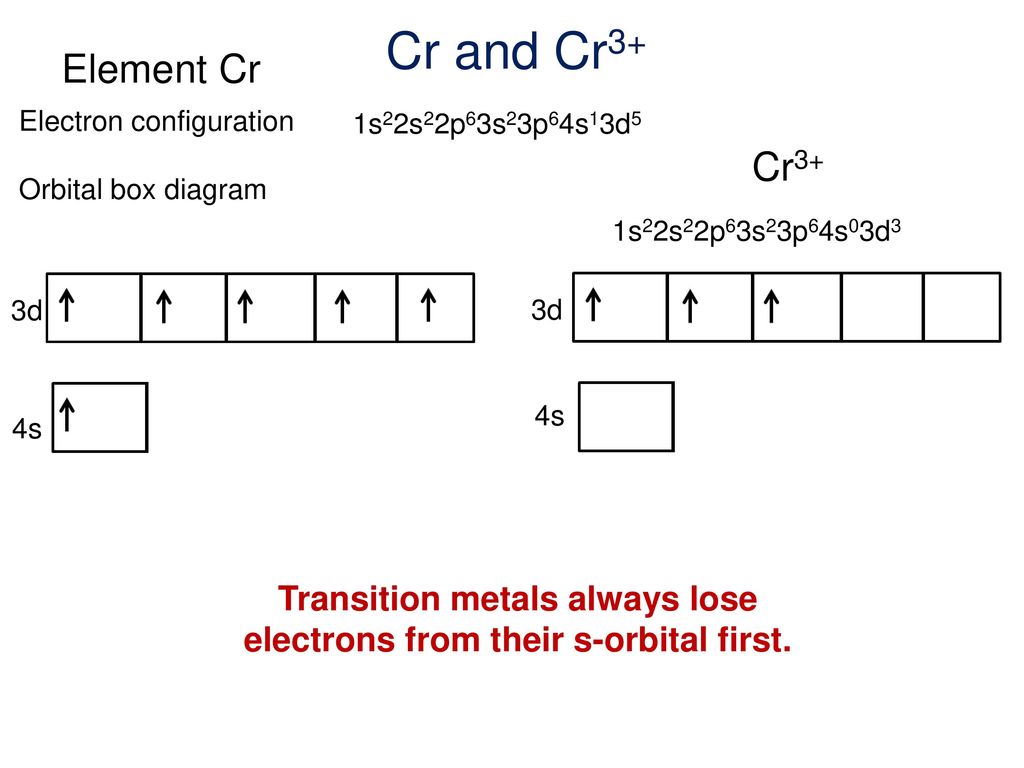
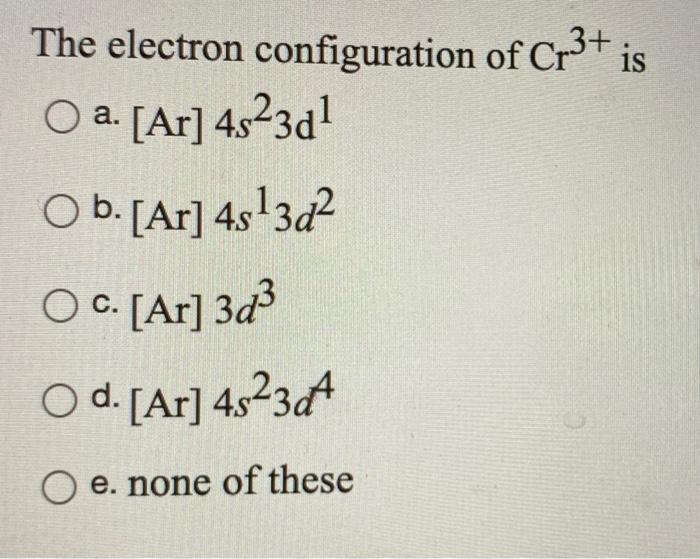
Orbital Diagram For Au+ - schematron.org The atomic number of Au is Therefore, its For Au+, one electron is removed from the outermost 6s orbital, making the configuration. Electron Configuration, [Xe] 4f14 5d10 6s1. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. Orbital Diagram. 1s. Electron Configuration for Chromium (Cr, Cr2+, Cr3+) Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules) In writing the electron configuration for Chromium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chromium go in the 2s orbital. The next six electrons will go in the 2p orbital. SOLVED:Write orbital diagrams for each ion and determine ... take a look here at utilizing the periodic table too. Do some orbital configurations or diagrams for some metal cat ions. So first example is going to be for chromium three plus. So as we follow the periodic table and fill the electrons properly, first row of the periodic table. Um we feel the first two electrons in the structure in the one S sub shell with its one orbital. What is the electron configuration of Cr 3+? | Socratic Note that it is 4s13d5 and not 4s23d4 because a half filled d orbital is more stable than a partially filled d orbital. However, the chromium ion Cr3+ possesses 24e− −3e− = 21e− due to the loss of 3 of its electrons. Thus, the electron configuration of Cr3+ is: Cr3+:1s22s22p63s23p64s03d3. Answer link.
V5+ Orbital Diagram - schematron.org A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. An orbital diagram naturally leads to the writing of an electron configuration. SOLVED:Write orbital diagrams for each ion and determine ... Do some orbital configurations or diagrams for some metal cat ions. So first example is going to be for chromium three plus. So as we follow the periodic table and fill the electrons properly, first row of the periodic table. Um we feel the first two electrons in the structure in the one S sub shell with its one orbital. Choose An Option That Best Describes Your Problem - BYJUS The electronic configuration of Cr having atomic number of 24 is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5 which is half-filled d-orbital.. Cr3+ has 3 electrons removed from the outermost shell. Therefore, the electronic configuration comes out to be [Ar]3d3. Electron Configuration for Cr, Cr2+, and Cr3+ (Exception ... Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules) Watch later. Share. Copy link. Info. Shopping. Tap to unmute. If playback doesn't begin shortly, try restarting your device.
Chromium(Cr) electron configuration and orbital diagram Atomic Orbital Diagram for Chromium (Cr) Chromium ion (Cr 2+, Cr 3+) electron configuration Ground state electron configuration of chromium (Cr) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 1. The electron configuration shows that the last shell of chromium has an electron and the d-orbital has a total of five electrons.
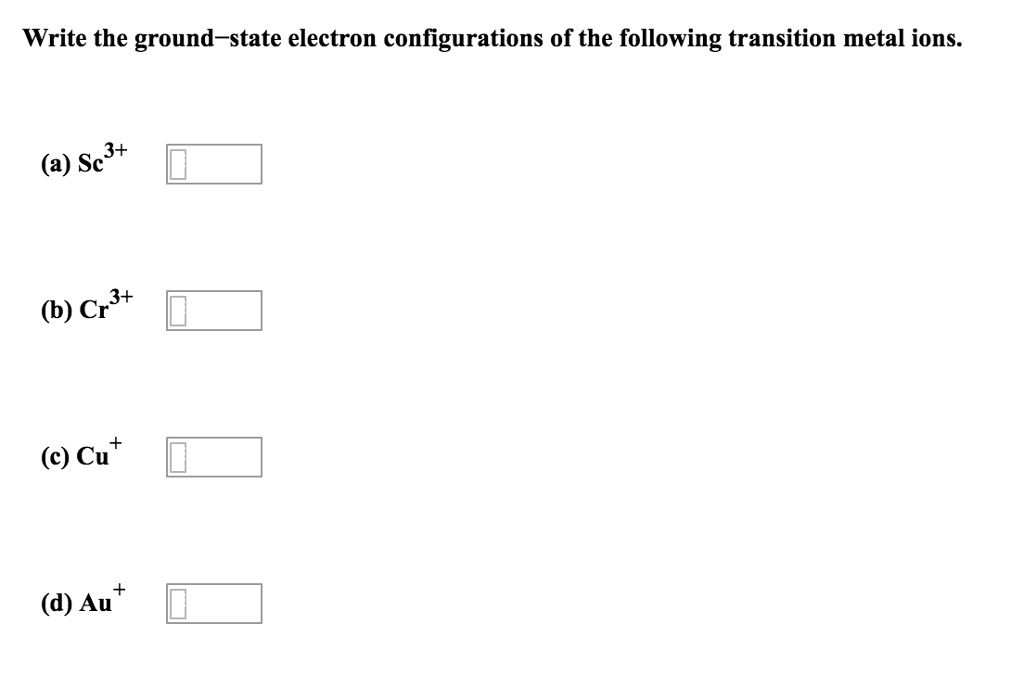
Write orbital diagrams for each ion and indicate whether ... Explanation. Step 1. 1 of 6. In writing orbital diagrams, first, determine the electron configuration of the neutral atom and remove electrons accordingly. Diamagnetic ions contain no unpaired electrons. Paramagnetic ions have unpaired electrons. Determine the orbital diagrams of a. V. 5 + ^ {5+} 5 +.
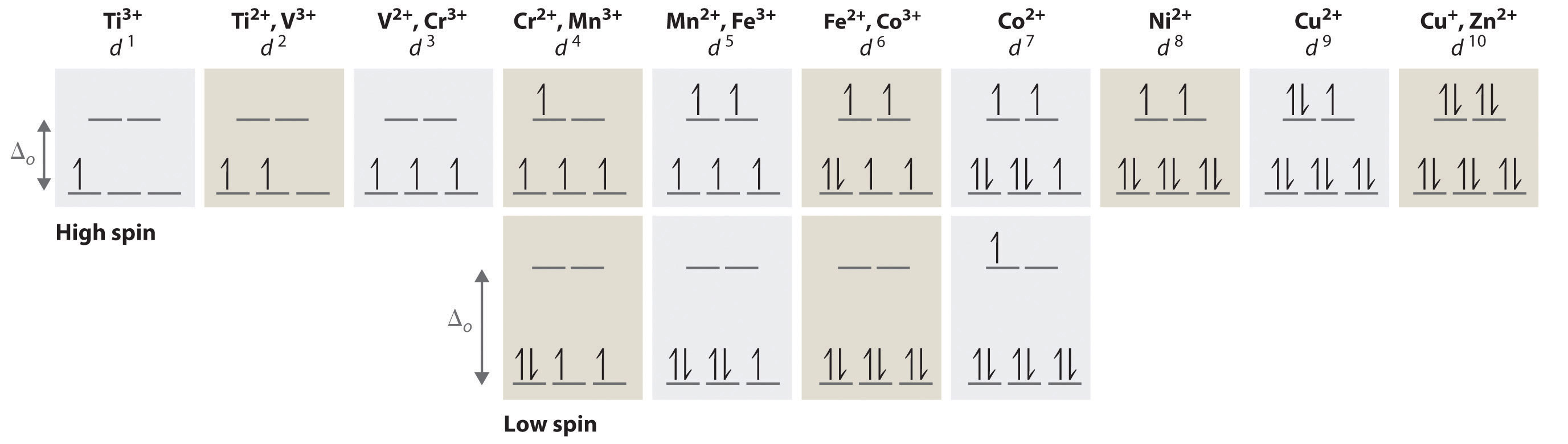
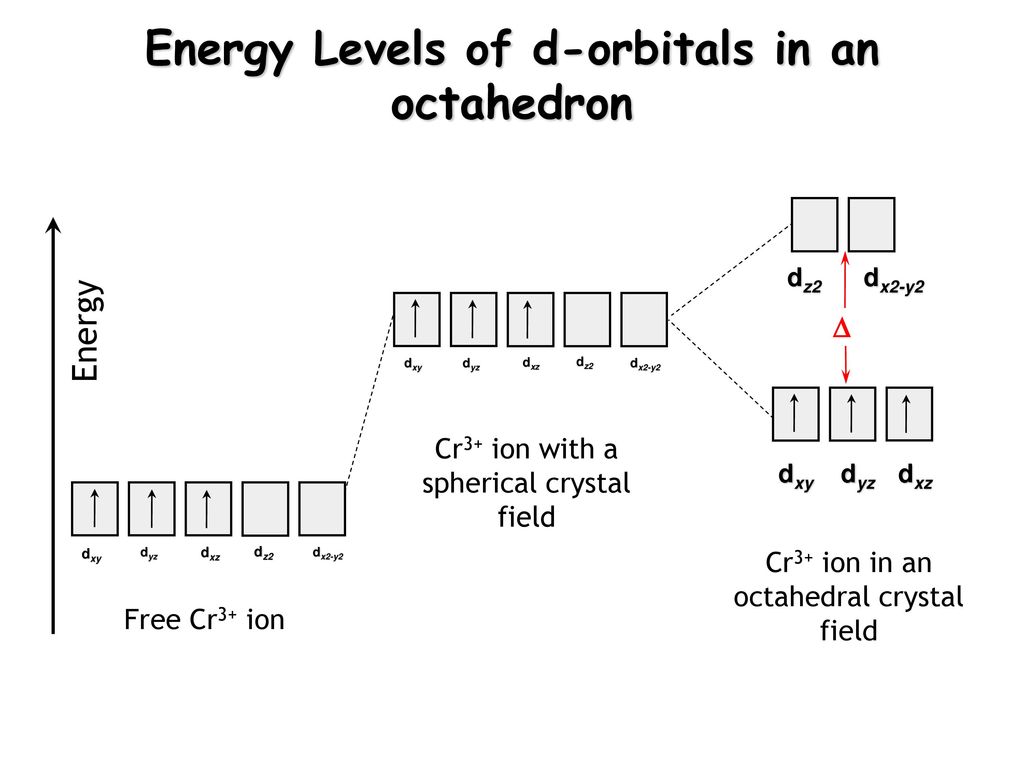
SOLVED:Draw the octahedral crystal field splitting diagram ... Ordeal Atrous left. So that's how Hey hi. Spin crystal filled orbital spitting diagram for em in Tripolis looks like and then for if it topless we has we have ah ah formal alike this hand in argon and then three d six so for this case, we have to find out the low spin or without spitting. So this is a law spin.
Molecular Orbital Theory, Integrated Rate Laws, The ... In today's live show I'll be going over: - Molecular Orbital Theory- Integrated Rate Laws- The Arrhenius Equation- Stoichiometry Word Problems📗 FREE CHEMIST...
40 co3+ orbital diagram For the carbonate ion, CO3 2−. 1- Draw the electron orbital diagram for the valence electrons of the central carbon. before and after hybridization. 2- Identify which carbon and oxygen electron orbitals overlap to create each single and double C-O bond in the structure. check_circle.
PDF MO Diagrams for More Complex Molecules • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection.
Mo3+ Orbital Diagram - Wiring Diagrams The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4.
Answered: Write orbital diagrams for each ion and… | bartleby Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. a. V5 + b. Cr3 + c. Ni2 + d. Fe3 +.
Answered: 6) Using arrows to represent electrons,… | bartleby 6) Using arrows to represent electrons, Construct the orbital diagram of the Cr and Cr3+ ion. Cr Cr3+ Determine which one has the larger radius. (Select one answer) 7) O Ca Ca2+ OMg2+ Sr2+ 8) Amongst those four elements, which one does not follow the general electron affinity trend: (Select one answer) ON OF ఐ Question Need solution urgently
What is the orbital diagram for chromium? - Quora commanly d-subshell have 5-d orbitals. each one orbital contain 2 electrons. so, totally 10 electrons. Half filled d5 & full filled d10 orbitals are more stable so, E.C of cr (24) is writen as [Ar]3d5 4s1 instead of [Ar]3d4 4s2 because half filled E.C is more stable. 5.8K views View upvotes Related Answer Aryan Jha
PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals?


![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure ...](https://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-5.png)

















0 Response to "39 orbital diagram for cr3+"
Post a Comment