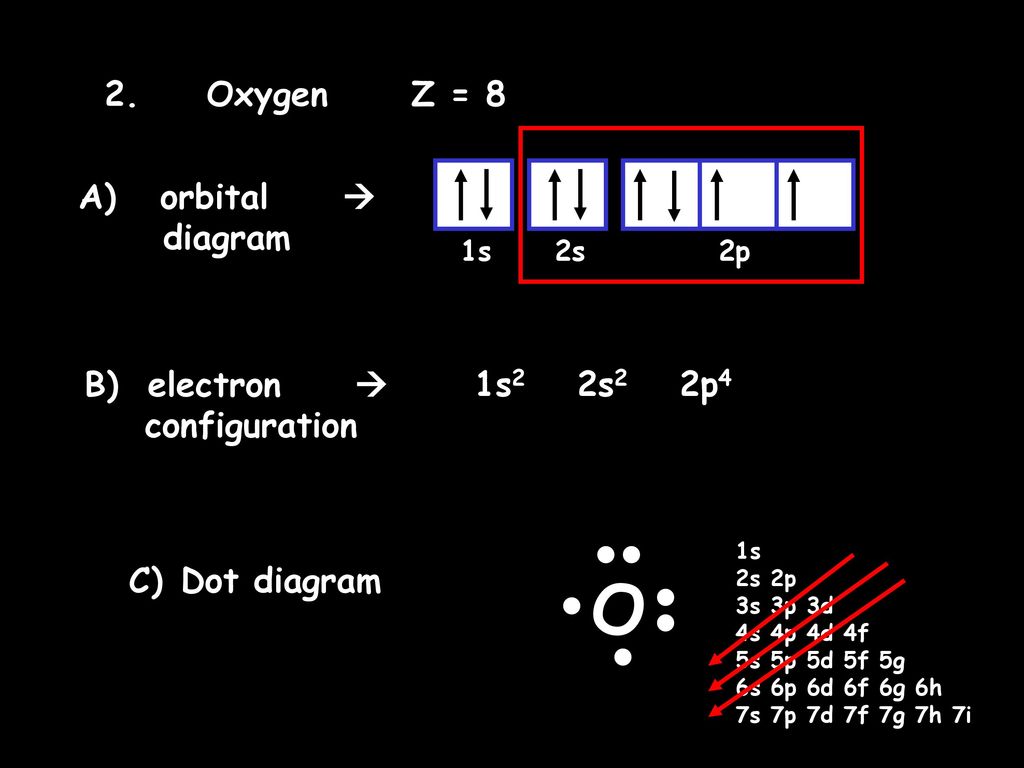
40 orbital filling diagram for oxygen
Orbital Diagrams Chemistry Tutorial How to draw orbital diagrams by applying Aufbau, Hund's and Pauli Principles tutorial with worked examples for chemistry students. So we draw the required orbital boxes for the orbital diagram using the Aufbau Principle to get the correct order for filling the boxes Oxygen(O) electron configuration and orbital diagram Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit] K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2. For example, 1. n = 1 for K orbit. The electron holding capacity of K orbit is 2n2 = 2 × 12= 2 electrons. 2. For L orbit, n = 2. The electron holding capacity of the L orbit is 2n2 = 2 × 22= 8 electrons. 3. n=3 for M orbit. The maximum electron holding capacity in M orbit is 2n2 = 2 × 32 = 18 electrons. 4. n=4 for N orbit. The maximum electron holding capacity in N orbit is 2n2 = 2 × 42= 32 electrons. Therefore, the maximum electron holding capacity in the first shell...
How to Do Orbital Diagrams | Sciencing Orbital diagrams give you all of the information you need about the electron configuration and occupied spin states for chemistry or physics, and See Resources for a diagram showing the filling order. Note that the n = 1 level only has s orbitals, the n = 2 level only has s and p orbitals, and the n...

Orbital filling diagram for oxygen
Orbital Diagrams & Electron Configurations for Atoms and Ions Orbital filling order for elements beyond Period 2 … ...corresponds to atom's location in periodic table! Draw an orbital diagram for beryllium (Z=4) 1s Guidelines for drawing 1s 2s 7 N nitrogen 14.01 2p Ti 8 O oxygen 16.00 Orbital diagrams for ions • anion (negative charge): ADD appropriate number... High School Chemistry/Orbital Configurations - Wikibooks, open books... The orbital diagram on the left is the correct orbital diagram, because it obeys Hund's Rule The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in (d) an oxygen atom has ___ unpaired non-valence electrons. Draw the orbital diagram for neon, Ne. Solved Which orbital-filling diagram represents the... | Chegg.com Transcribed image text : Which orbital-filling diagram represents the ground state of oxygen? They are usually only set in response to actions made by you which amount to a request for services, such as setting your privacy preferences, logging in or filling in forms.
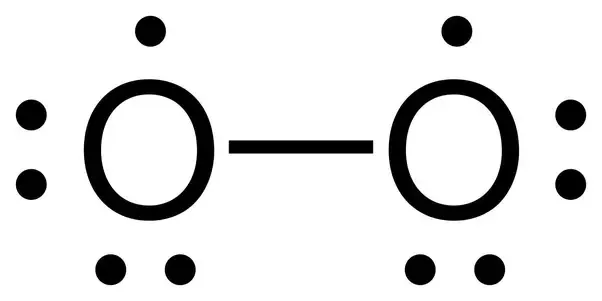
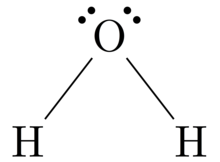
Orbital filling diagram for oxygen. Draw the orbital diagram for oxygen Draw the molecular orbital diagram for oxygen molecule. Learning objective to draw, interpret and convert between Lewis (Kekule), condensed and bond line structures Note: The revision of the general chemistry in the Sections 1.3 - 1.6 is integrated into the learning goal above for the chemical organ ¢ Nica in the Sections 1.7 and 1.8. The Orbital Diagram For A Ground State Oxygen Atom Is The orbital diagram for a ground state oxygen atom is a a b b c c d d e e which element has the following ground state electron configurati... Chapter 5 Orbital Filling Diagrams and Electron Dot Diagrams. 7 Orbital Filling Diagrams. 8 A. General Rules Pauli Exclusion Principle -Each orbital can hold TWO electrons with opposite spins. 11 What type of sublevel is this? 12 Make the orbital filling diagram for d 8 How many of the electrons are unpaired? 18 Draw the electron dot diagram for oxygen. Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Electron Configurations - Course Hero In the orbital box diagram for oxygen, the electrons in the 2p degenerate orbital fill out as singletons before doubling up with an electron of opposite spin. According to Hund's rule, when filling orbitals of the same energy, electrons occupy empty orbitals before doubly occupying the same orbital. PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... • Can represent p orbital as dot diagrams or boundary surfaces. • Electrons are filled in increasing energy (Aufbau principle) and electrons fill degenerate (same energy) levels singularly first to give - Oxygen - hydrogen interactions share 2 electron Æ H ─ O. - Oxygen also has two lone pairs. Orbital Filling Diagram For Nitrogen - Drivenhelios Show the orbital filling diagram for rm n nitrogen best answer the electronic configuration for nitrogen atom is 1s 2 2s 2 2p 3 lowest energy state will What Is The Molecular Orbital Diagram For Oxygen Quora. Electron Configurations The Periodic Table. 12 9 Orbital Shapes And Energies Chemistry... How To Draw Molecular Orbital Diagram Of Co - Drawing ... Sep 27, 2021 · Molecular orbital diagram for oxygen gas (o2).fill from the bottom up, with 12 electrons total.bonding order is 2, and it is paramagnetic.sigma2s(2),sigma2s*. Building molecular orbital diagrams for homonuclear and heteronuclear diatomic molecules; (1) e n = 13.6 z e f f 2 n 2 e v.
s p d f obitals notation shapes diagrams how to work out electron... s atomic orbital diagram. s orbitals have a spherical shell shape and the faint dark blue circle sp d f orbital notation are used in writing out electron configurations for chemical elements and their ions. How do you work out the electron arrangement configuration for 8 Oxygen, O ? Figure 4. Orbital filling diagram for oxygen. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Each sublevel is labeled by its... 8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Each oxygen atom contributes six electrons, so the diagram appears as shown in Figure 8.40. Figure 8.40 The molecular orbital energy diagram for O2 predicts two unpaired electrons. What is the orbital diagram for oxygen? - Answers Earn +20 pts. Q: What is the orbital diagram for oxygen? The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons.
What is the molecular orbital diagram for oxygen? - Quora From the molecular orbital diagram, we observe that oxygen has two unpaired electrons which is consist with the paramagnetic nature of oxygen. The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in...
Orbital filling diagrams | The Cavalcade o' Chemistry Feb 23, 2016 · The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there’s a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
How to Write the Orbital Diagram for Oxygen (O) - YouTube To write the orbital diagram for the Oxygen atom (O) first we need to write the electron configuration for just O. To do that we need to find the number of ...
8.4 Molecular Orbital Theory - Chemistry The filled molecular orbital diagram shows the number of electrons in both bonding and Figure 9. The molecular orbital energy diagram predicts that H2 will be a stable molecule with lower Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from...
How do yo write the orbital diagram for oxygen? | Socratic The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram. The video uses Kr as an example, but the process is exactly as the same as what you need to do for oxygen. Hope this helps!
Oxygen | The Periodic Table at KnowledgeDoor | Orbital Filling Order Our oxygen page has over 280 facts that span 108 different quantities. Each entry has a full citation identifying its source. [He] represents the closed-shell electron configuration of helium. Orbital Filling Order. Bulletin of Alloy Phase Diagrams, volume 3, number 2, 1982, pp. 275-276. doi:10.1007...
Molecular Orbital Theory: Energy level diagram for molecular orbitals (vii) The molecular orbitals are filled in the increasing order of their energies, starting with orbital of least energy. This energy diagram for the molecular orbitals is shown in Fig.1 However, experimental evidence for oxygen and heavier diatomic molecules have shown that above sequence...
Orbital-filling diagram - HomeworkLib Show the orbital-filling diagram for (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshellat 13. In polyatomic atoms electrons fill the orbitals from the lowest energy to the highest. Write the electron configuration of oxygen.
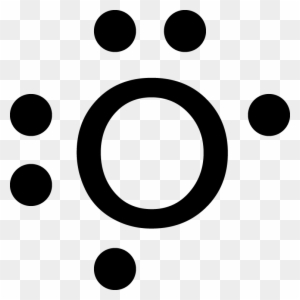
Energy Levels, Orbital Diagrams, Electron Config, Noble Gas When filling in orbital diagrams, no orbital may contain 2 arrows pointing in the same direction. Lets do some examples: Draw the orbital diagram for Oxygen.
orbital diagram flashcards and study sets | Quizlet Learn about orbital diagram with free interactive flashcards. O (Oxygen). What element is represented by this orbital diagram? Orbital filling diagram for carbon. electrons fill the lowest energy levels first.
Electron Configurations | Orbital Diagrams Based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. So Oxygen's electron configuration would be O Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent...
Atomic and Molecular Orbital Diagram for Oxygen/O2 Discuss: orbital energies, orbital filling, magnetism. molecular electron configurations. Compare: Sum of # of atomic orbitals = # of molecular orbitals. Remembering how to draw the MO diagram for B,C,N Vs O,F,Ne - using dry erase board, generic diagram handout, or other.
PDF Microsoft PowerPoint - Zumdahl9 For filled K shell bondin g and antibondin g orbitals use KK designation. This attraction of liquid oxygen for the magnetic field demonstrates the paramagnetism of the O2 molecule. Figure 9.41: The molecular orbital energy-level diagram for the NO molecule.
Orbital Diagrams — Overview & Examples - Expii An orbital diagram, or orbital filling diagram, is a type of notation which illustrates an atom's electron distribution and electron spin within orbitals. The Basics of Orbital Diagrams. There are different types of orbitals, that all have different energy levels. These orbitals are filled with electrons (the...
Making orbital filling diagrams - YouTube Electron configurations & electron configuration lecture for exp chem.
Solved Which orbital-filling diagram represents the... | Chegg.com Transcribed image text : Which orbital-filling diagram represents the ground state of oxygen? They are usually only set in response to actions made by you which amount to a request for services, such as setting your privacy preferences, logging in or filling in forms.
High School Chemistry/Orbital Configurations - Wikibooks, open books... The orbital diagram on the left is the correct orbital diagram, because it obeys Hund's Rule The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in (d) an oxygen atom has ___ unpaired non-valence electrons. Draw the orbital diagram for neon, Ne.
Orbital Diagrams & Electron Configurations for Atoms and Ions Orbital filling order for elements beyond Period 2 … ...corresponds to atom's location in periodic table! Draw an orbital diagram for beryllium (Z=4) 1s Guidelines for drawing 1s 2s 7 N nitrogen 14.01 2p Ti 8 O oxygen 16.00 Orbital diagrams for ions • anion (negative charge): ADD appropriate number...










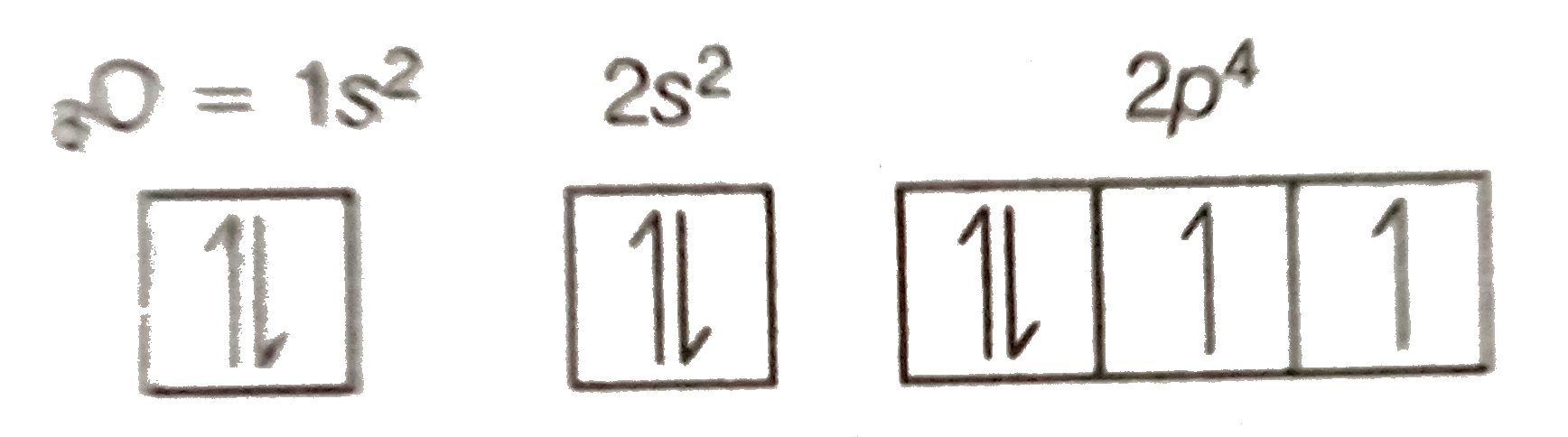














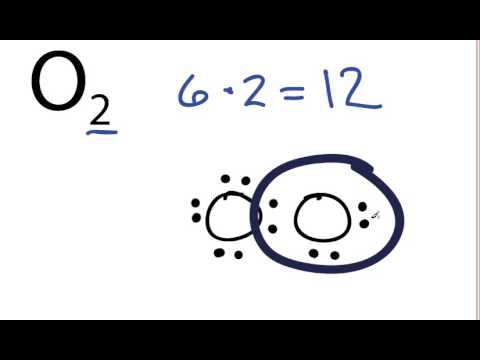




0 Response to "40 orbital filling diagram for oxygen"
Post a Comment