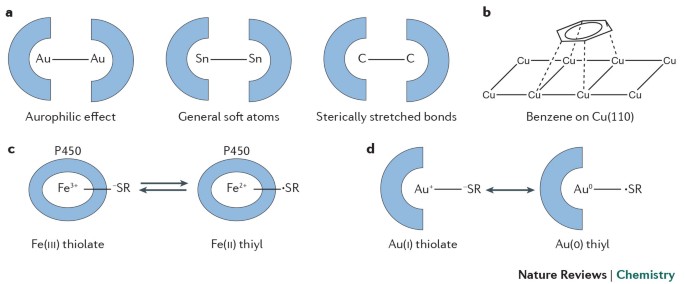
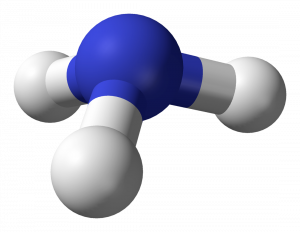
39 the ammonia molecule in the diagram has the observed bond orientation because
39 the ammonia molecule in the diagram has the observed bond ... Feb 26, 2022 · The ammonia molecule in the diagram has the observed bond orientation because. the ammonia molecule in the diagram has the observed bond orientation because. n has four pairs of electrons in the valence shellb. electrons repel one another c. rotation can occur around single bonds. all of the above e. n has 7 protons in its nucleus. Front view and side view of optimized structure of BN ... The relaxed structures for all the geometries with change in the bond lengths and the bond angles are shown in Fig. 3(a-d), where, it is observed that for all the positions, NH molecule prefers...
Molecular Interactions (Noncovalent Interactions) Figure 27 illustrates the hydrogen bonding as observed in crystalline ammonia. The hydrogen bonds are longer than those in ice and are non-linear. Although each ammonia molecule forms hydrogen bonds with six neighbors in the crystal, only two ammonia molecules are shown here.

The ammonia molecule in the diagram has the observed bond orientation because
Mastering Biology | Chapter 2 Key Concepts - Quizlet The ammonia molecule in the diagram has the observed bond orientation because... a) electrons repel one another b) N has four pairs of electrons in the valence shell c) N has 7 protons in its nucleus d) all of the above e) none of the above PDF Waals clusters J. A. Fernandez and E. R. Bernstein ... bare molecule.10 Large shifts in the cluster electronic transi-tion origin are thereby produced. In this paper we present a study of the aniline/ammonia cluster system for up to three ammonia molecules solvating the aniline molecule. This system is of particular interest because it suggests the possibility of three distinct limiting Molecular Structure and Polarity - Chemistry Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space ().A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees.
The ammonia molecule in the diagram has the observed bond orientation because. The ammonia molecule in the diagram has the observed bond ... 👍 Correct answer to the question The ammonia molecule in the diagram has the observed bond orientation because - e-eduanswers.com Ch. 2: Chemistry of Biology Flashcards - Quizlet The ammonia molecule in the diagram has the observed bond orientation because ... A. N has four pairs of electrons in the valence shell. B. electrons repel one another. C. N has 7 protons in its nucleus. D. All of the above. E. None of the above. 4.6 Molecular Structure and Polarity - Chemistry: Atoms ... Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space (Figure 4.14).A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. The ammonia molecule in the diagram has the observed bond ... Dec 16, 2019 · The shape and the bond orientation of molecules and ions are both explained by the valences shell electron pair repulsion theory (VSEPR). Ammonia, , is a molecule which contains three N-H bonds, as well as one lone pair on nitrogen. According to the VSEPR theory, molecules try to acquire a shape which would minimize the repulsion exhibited by ...
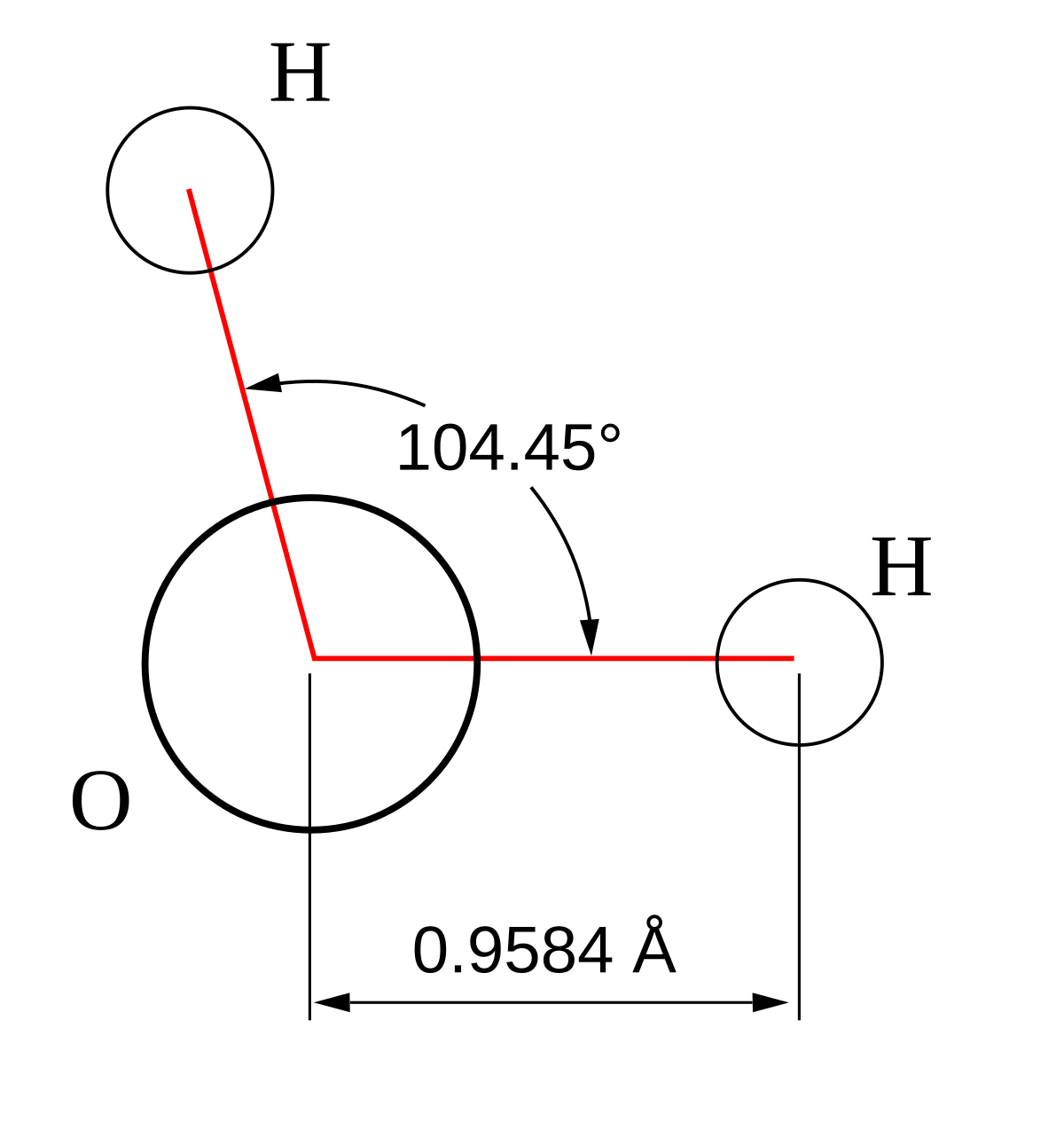
Formation of Ammonia molecule Archives - The Fact Factor Valence Bond Theory fails to explain the observed geometry of the molecules of water and ammonia e.g. in the formation of H 2 O molecule, the H - O - H bond angle should be 90°. But the measured bond angle is 104.3° and the molecule is V-Shaped. Valence bond theory failed to explain this change. Mastering Biology Set 1 Flashcards | Chegg.com electrons in two orbitals. The ammonia molecule in the diagram has the observed bond orientation because ... N has 7 protons in its nucleus. AND electrons repel one another. AND N has four pairs of electrons in the valence shell. Without making or breaking bonds, the pictured molecule can change its shape because ... Mastering Biology | Chapter 2 Key Concepts - Chegg The ammonia molecule in the diagram has the observed bond orientation because... a) electrons repel one another b) N has four pairs of electrons in the valence shell c) N has 7 protons in its nucleus d) all of the above e) none of the above AP Bio Chapter 2 Flashcards - CourseNotes The ammonia molecule in the diagram has the observed bond orientation because ... a. N has four pairs of electrons in the valence shell b. electrons repel one another c. N has 7 protons in its nucleus d. All of the above e. None of the above: All of the above Since N has 7 protons, it must fill the second shell, giving it 4 pairs of electrons.
The Chemical Bond - Molecular Geometry All we demand of our simple theory is that it correctly predict whether the water molecule is linear (bond angle = 180°) or bent (bond angle less than 180°) Or as another example, it should predict whether the ammonia molecule is planar (a) or pyramidal (b). The observed geometry of a molecule is that which makes the energy of the system a minimum. Chemisorption of Carbon-Monoxide and Ammonia on Nickel and ... The process of chemisorption of small molecules on transition metal surfaces has been investigated from the point of view of two prototype adsorption systems, carbon monoxide on iridium {100} and {111 } and ammonia on nickel {111}. Microscopic details such as the molecular orientation on the surface, the bonding configuration and the nature of interactions between adsorbed species have been ... Does Ammonia Hydrogen Bond? - JSTOR hydrogen bond, and in that case alone this simple hydrogen-bonding model fails to predict the experimental structure. In Fig. 1 the observed hydrogen-bonding lengths are also displayed; the HF hydrogen bonds are shortest (and presumably strongest) and range from 1.78 to 1.83 A, whereas the H20 hydrogen bonds vary from 2.02 to 2.05 A. 31 The Ammonia Molecule In The Diagram Has The Observed ... The ammonia molecule in the diagram has the observed bond orientation because. the ammonia molecule in the diagram has the observed bond orientation because. n has four pairs of electrons in the valence shellb. electrons repel one another c. rotation can occur around single bonds. all of the above e. n has 7 protons in its nucleus.
MasteringBiology Chapter 2 - Subjecto.com The ammonia molecule in the diagram has the observed bond orientation because … N has 7 protons in its nucleus. N has four pairs of electrons in the valence shell. electrons repel one another. All of the above. None of the above. All of the above. Without making or breaking bonds, the pictured molecule can change its shape because …
M12Q1: Refresher of VSEPR, VBT, and Polarity in ... - Unizin (a) Each CO bond has a bond dipole moment, but they point in opposite directions so that the net CO 2 molecule is nonpolar. (b) In contrast, water is polar because the OH bond dipole moments do not cancel out. The OCS molecule has a structure similar to CO 2, but a sulfur atom has replaced one of the oxygen atoms. To determine if this molecule ...
PDF 1 Theories in conflict - Wiley Start with the observed structure and use this to obtain information about the bonding. But first, the more traditional approach. 1.2 The ammonia molecule The ammonia molecule provides a convenient starting point for our study and it will be used to see the problem of chemical bonding in a rather unusual perspective, one that leads
Lutron Three Way Switch Wiring Diagram - schematron.org Alpine 445u Wiring Diagram; Isuzu I290 Engine Wiring Diagram; Ge Gss22wg Wiring Diagram; Cadette Sash Diagram; Intermatic 240v Timer Wiring Diagram; The Ammonia Molecule In The Diagram Has The Observed Bond Orientation Because ... Ouku Radio Wiring Diagram; Dual Xhd6425 Wiring Harness; Recent Comments. Brad H. on Lutron three way switch wiring ...
The ammonia molecule, with the H-N-H angle, N-H bond ... To obtain a more accurate expression of the rate for the special case of ammonia molecule, we observed geometry optimization which leads to N-H bond length of 1.017 Å, while H-N-H bond angle is ...
Chemistry Review - Atoms & Molecules: Covalent Bonds ... The ammonia molecule in the diagram has the observed bond orientation because … There is a ball-and-stick model of ammonia, NH3. Three hydrogen atoms are attached to nitrogen. ANSWER: N has four pairs of electrons in the valence shell. N has 7 protons in its nucleus. electrons repel one another. All of the above. None of the above. Part G
The Ammonia Molecule In The Diagram Has The Observed Bond ... Dec 25, 2018 · The ammonia molecule in the diagram has the observed bond orientation because a. (c) The electrostatic potential diagram of the water molecule. The polarity of the NOH bonds occurs because nitrogen has a greater electronegativity than hydrogen. (b) The dipole moment of the ammonia molecule oriented in an electric field.
The ammonia molecule in the diagram has the observed bond ... The ammonia molecule in the diagram has the observed. This preview shows page 1 - 3 out of 4 pages. The ammonia molecule in the diagram has the observed bond orientation because…. All of the above (N has 7 protons in its nucleus, electrons repel one another, N has four pairs of electrons in the valence shell) The discovery of which of the ...
Nitrogen Molecule - an overview | ScienceDirect Topics The left picture marks three coordinates z using vertical dotted lines which correspond to the orientation distributions o(θ, ϕ) a), b) and c). cos(θ) = 1 corresponds to the molecular orientation perpendicular to the z-axis of the pore. cos(ϕ) = 1 means that a molecule has been rotated along π/2 out of the x-z-plane around that z-axis of ...
The ammonia molecule in the diagram has the observed bond ... Dec 17, 2019 · Ammonia, , is a molecule which contains three N-H bonds, as well as one lone pair on nitrogen. According to the VSEPR theory, molecules try to acquire a shape which would minimize the repulsion exhibited by the electron clouds present, that is, between the bonding (shared in a bond) and non-bonding (lone pair) electrons.
7.6 Molecular Structure and Polarity - Chemistry (a) Each CO bond has a bond dipole moment, but they point in opposite directions so that the net CO 2 molecule is nonpolar. (b) In contrast, water is polar because the OH bond moments do not cancel out. The OCS molecule has a structure similar to CO 2, but a sulfur atom has replaced one of the oxygen atoms. To determine if this molecule is ...
Mastering Biology Chapter 2.docx - Mastering Biology Chapter ... 49) The ammonia molecule in the diagram has the observed bond orientation because ... N has 7 protons in its nucleus, N has 4 pairs of electrons in the valence shell, and electrons repel one another 50) Without making or breaking bonds, the pictured molecule can change its shape because ... rotation can occur around single bonds.
Part D By making two covalent bonds an O atom with 8 ... ANSWER: The ammonia molecule in the diagram has the observed bond orientation because ... N has 7 protons in its nucleus. N has four pairs of electrons in the valence shell. electrons repel one another. All of the above. None of the above. Part G The efficiency of any engine is , by definition , .
Molecular Structure and Polarity - Chemistry Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space ().A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees.
PDF Waals clusters J. A. Fernandez and E. R. Bernstein ... bare molecule.10 Large shifts in the cluster electronic transi-tion origin are thereby produced. In this paper we present a study of the aniline/ammonia cluster system for up to three ammonia molecules solvating the aniline molecule. This system is of particular interest because it suggests the possibility of three distinct limiting
Mastering Biology | Chapter 2 Key Concepts - Quizlet The ammonia molecule in the diagram has the observed bond orientation because... a) electrons repel one another b) N has four pairs of electrons in the valence shell c) N has 7 protons in its nucleus d) all of the above e) none of the above
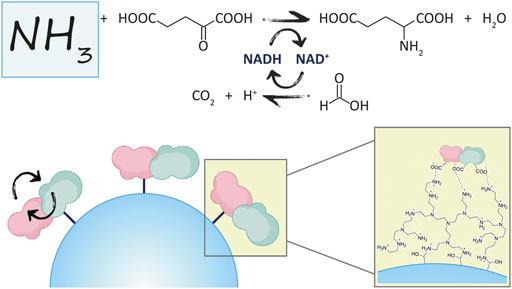




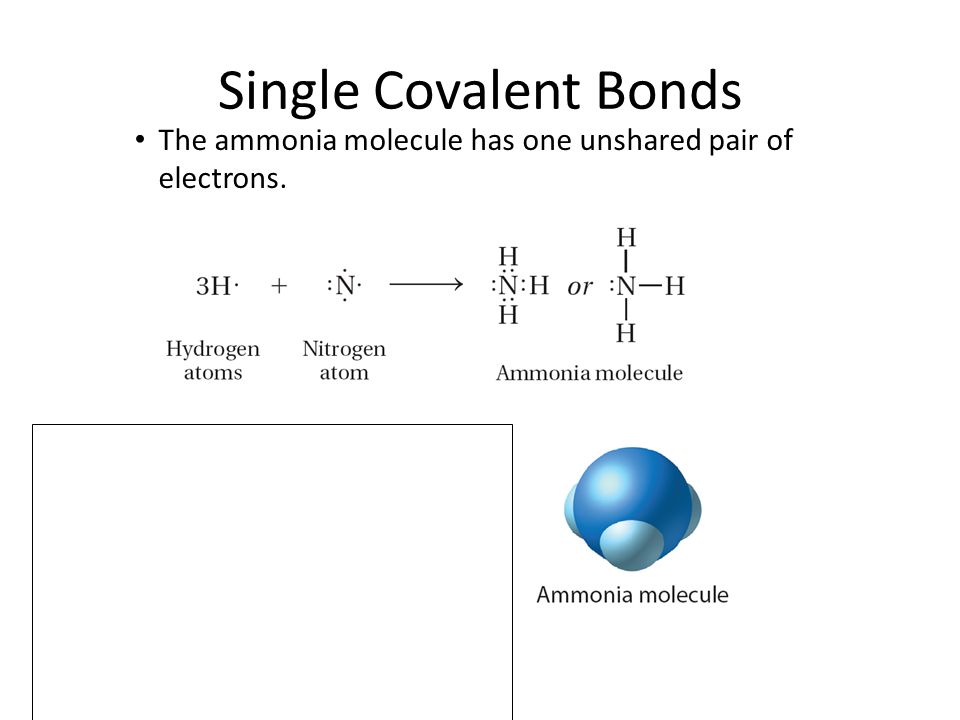

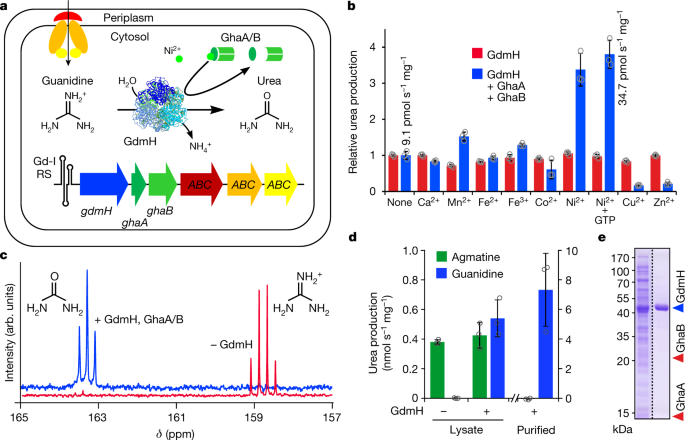















0 Response to "39 the ammonia molecule in the diagram has the observed bond orientation because"
Post a Comment