38 lewis dot diagram of nitrogen
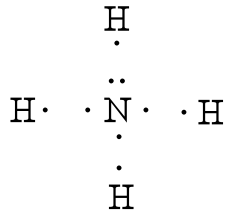
What is Lewis dot diagram of nitrogen gas? - Answers The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. .. . N . . Lewis Structures (electron dot diagrams) Chemistry Tutorial Lewis Structure (electron dot diagram) for ammonia OR Note that there are 3 covalent bonds (3 bonding pairs of electrons) in total, and that there is a lone pair (non-bonding pair) of electrons on the nitrogen atom.
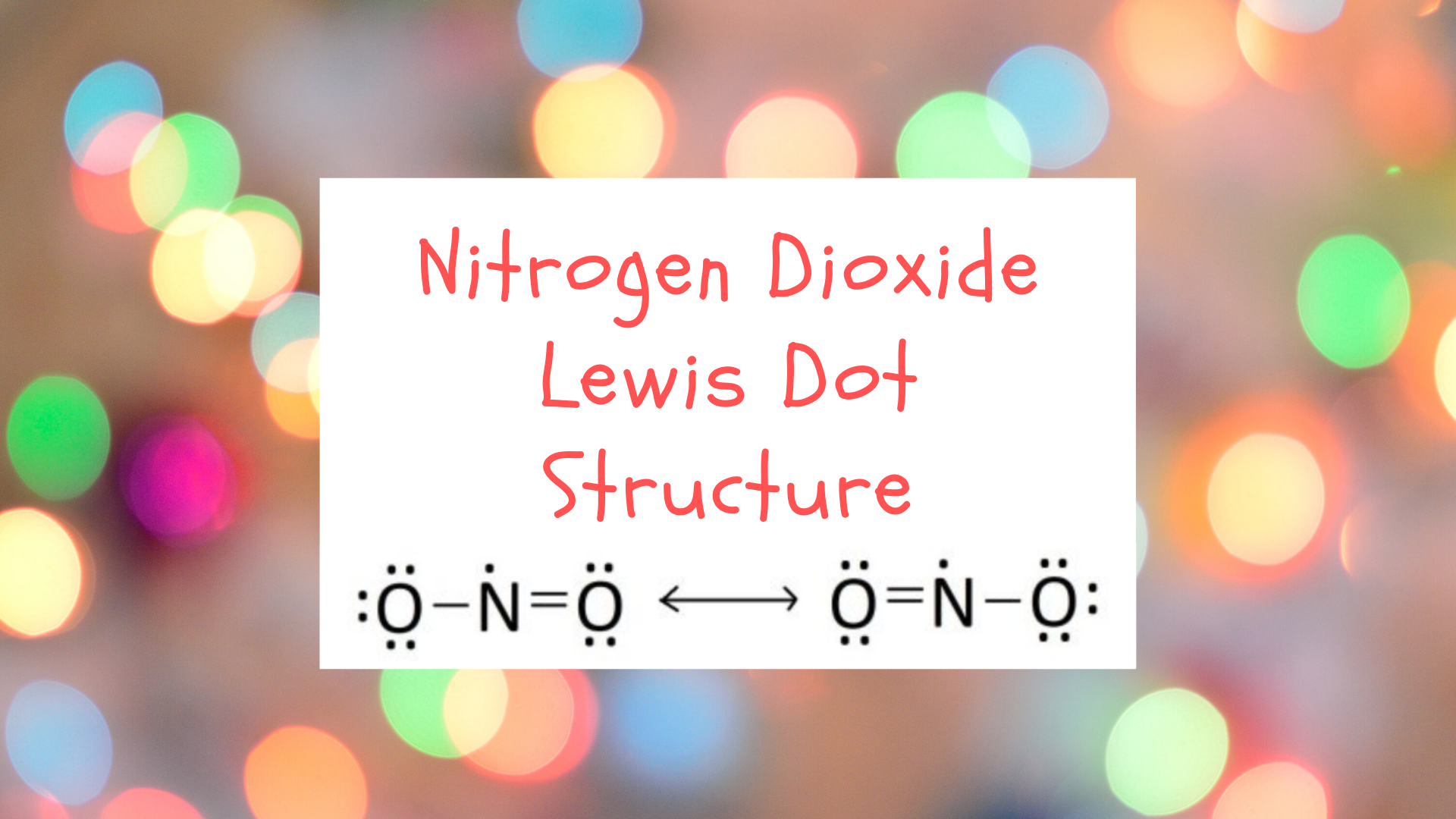
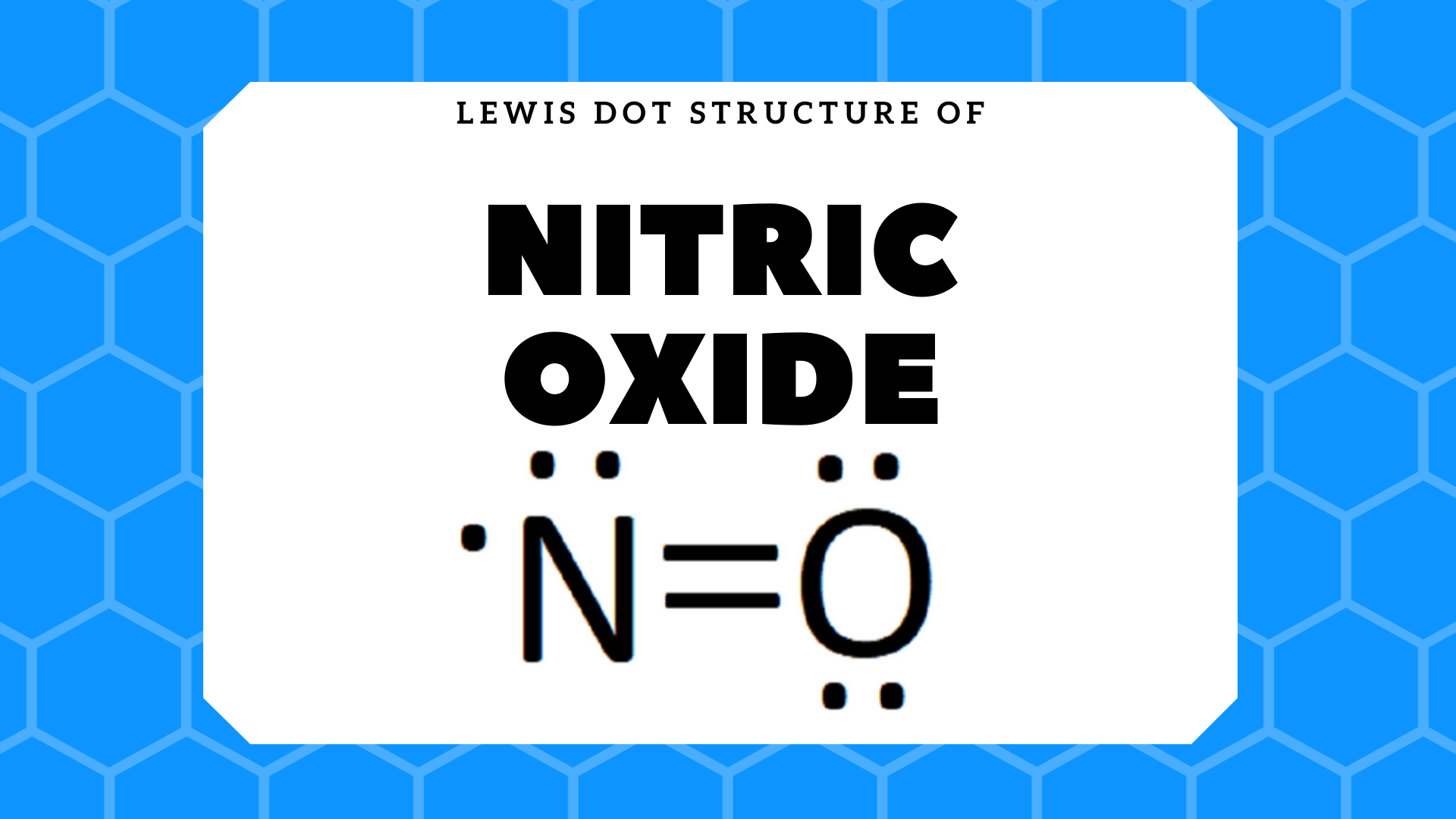
NO2 (Nitrogen Dioxide) Lewis Dot Structure | Science Trends Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3.It is slightly toxic to humans, on account of its tendency to react in the human body and produce reactive species of nitrogen and ...

Lewis dot diagram of nitrogen
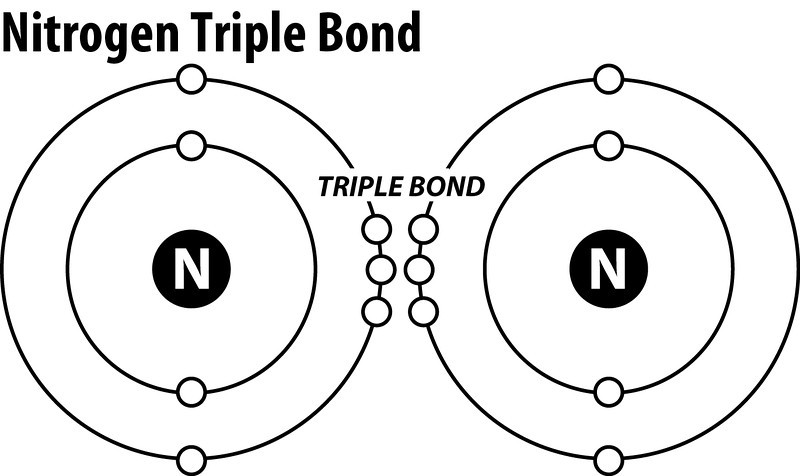
What is the Lewis dot structure for nitrogen gas ... What is the Lewis dot structure for nitrogen gas? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable. NCl3 (Nitrogen trichloride) Lewis Structure Nitrogen trichloride (NCl3) lewis structure contains three N-Cl bonds. There is one lone pair on nitrogen atom and three lone pairs on each chlorine atom. Lewis structure of NCl3 can be drawn by using valence electrons of nitrogen and chlorine atoms. Also, there are no charges on atoms in NCl3. Steps of drawing the lewis structure of NCl3 are explained in detail in this tutorial. Lewis Electron Dot Diagrams - Introductory Chemistry - 1st ... A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. ... With nitrogen, which has three p electrons, we put a single dot on each ...
Lewis dot diagram of nitrogen. What is the electron dot diagram for nitrogen? Note: Nitrogen is in Group 5 (sometimes called Group V or Group 15). Since it is in Group 5 it will have 5 valence electrons. When you draw the Lewis structure for Nitrogen you'll put five "dots" or valance electrons around the element symbol (N). Click to see full answer Similarly, what are electron dot diagrams used for? Lewis Dot Structure For Nitrogen Disulfide - Novocom.top lewis dot electron diagram structure nitrogen atom diagrams dots bismuth electrons valence five arrangement form . n2 lewis structure nitrogen gas draw . ã 2 Stepsã N2 î Lewisî î Structureî î Lewisî î Dotî î Structureî for . Draw and explain the Lewis dot structure of nitrogen ... Lewis dot structure is a diagram that represents the number of valence electrons of an element through dots around the element symbol. Learn about Lewis dots and understand single bonds, double ... Lewis Dot Diagram For N2 - schematron.org on Lewis Dot Diagram For N2. Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple. The Lewis Structure for N2 looks easy at first. The problem is that there aren't enough valence electons to give both Nitrogen atoms an octet. You'll need to use .
PDF Atomic Protons Neutrons Electrons Lewis Dot Mass Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li Lewis Dot Structures - Definition and Example | Chemistry Lewis dot structure is also known by some other names such as electron dot structure, Lewis electron-dot formula, or Lewis Electron Dot Structures (LEDs), etc. It has many applications in Chemistry, especially for the topics related to the study of bonds between atoms, the atomic structure of atoms, molecular & organic Chemistry, etc. Lewis Dot Structure for Nitrogen Atom (N) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for N (Nitrogen). I show you where Nitrogen is on the periodic table and how to determine ... N2H4 Lewis Structure, Geometry, Hybridization, and ... Lewis Structure of N2H4 Lewis dot diagram or electron dot structure is the pictorial representation of the molecular formula of a compound along with its electrons that are represented as dots. These structures are named after American chemist Gilbert Newton Lewis who introduced them in 1916.
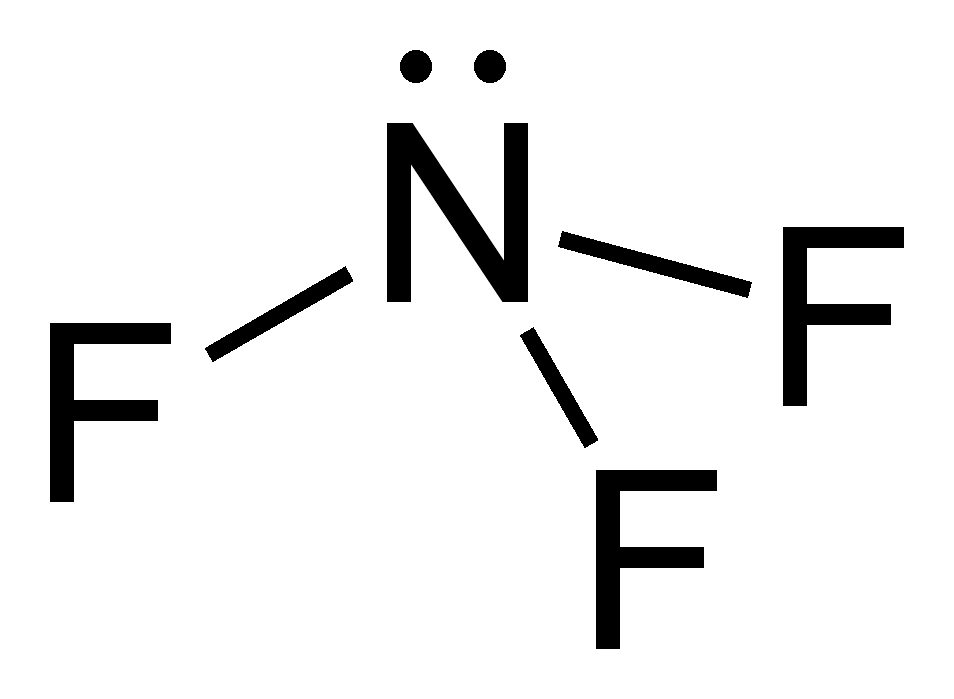
N2 Lewis Structure: Full Guide (2022 Updated) Steps In Drawing the N2 Lewis Structure To create a Lewis structure, determine first the number of valence electrons in each atom. Nitrogen has a total of ten valence electrons—five electrons on its outermost valence shell. After determining the total number of valence electrons., connect the atoms between electron pairs. Nitrogen trifluoride (NF3) lewis dot structure, molecular ... So, nitrogen is the central atom that has 1 lone pair and 3 bonded pair electrons according to the NF3 lewis dot structure. Hence the formula of NF3 becomes AX3N1 So, according to the VSEPR chart, if the molecule has the formula of AX3N1, it has a molecular shape of trigonal pyramid and electron geometry of tetrahedral. How to Draw the Lewis Dot Structure for N2: Nitrogen Gas ... A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t... what is the electron dot structure for calcium ... Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. … Electron dot diagrams would be the same for each element in the representative element groups.
N2 Lewis Structure, Molecular Geometry, and Hybridization ... Steps to Draw the Lewis structure of N2 Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell. As per the molecule N2, it has two atoms of Nitrogen.
Nitrogen (N2) Molecule Lewis Structure Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure. N 2 lewis structure There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.
What is the Lewis dot structure for nitrogen ... What is the dot structure of NO2? The NO2 Lewis structure has a total of 17 valence electrons. It's not common to have an odd number of valence electrons in a Lewis structure. Because of this we'll try to get as close to an octet as we can on the central Nitrogen (N) atom.
NO2 Lewis Structure: Complete Guide (2022 Updated) Understanding The NO2 Lewis Structure One nitrogen atom and two oxygen atoms make up nitrogen dioxide. [1] Nitrogen belongs to group 15 elements in the periodic table with five valence electrons, while the oxygen atom has six electrons. The nitrogen atom is in the center, surrounded by Oxygen atoms.
Nitrogen trichloride (NCl3) lewis dot structure, molecular ... Lewis diagram is a representation of how electrons are arranged around individual atoms in a structure. NCl3 lewis structure is the same as the NF3 structure. It contains one nitrogen atom at the center and three chlorine atoms spaced evenly around it.
Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules. 3. Determine the electron and molecular geometry of the produced molecules. Background: Scientists often create models to represent either a physical or ...
Bohr Rutherford Diagram For Nitrogen A Lewis dot structure is like a simplified Bohr-Rutherford model. The Lewis Dot diagram contains the . Encyclopedia Britannica explains that a Bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete energy quanta.
PDF Periodic Table, Valence Electrons & Lewis Dot Diagrams ELECTRONS & LEWIS DOT DIAGRAMS Na 11 22.9898 sodium Remember that from the periodic table we can determine the number of protons, neutrons & electrons of any element! ... Draw a Bohr Diagram for nitrogen in your notes! PERIODIC TABLE, VALENCE ELECTRONS & LEWIS DOT DIAGRAMS Lewis Dot Diagram Shows on the valence electrons
Lewis Dot Diagram - Organic Chemistry - Socratic Nitrogen, Group V, has 5 valence electrons; oxygen, Group VI, has 6 valence electrons. And we throw in another electron, so that we have 5 +3 ×6 + 1 = 24 valence electrons, i.e. 12 electron pairs in the Lewis structure of N O− 3 to distribute around 4 centres. And thus we get O = N +( −O−)2.
What is the electron dot diagram for nitrogen? When you draw the Lewis structure for Nitrogen you'll put five "dots" or valance electrons around the element symbol (N). In respect to this, what are electron dot diagrams used for? There are shorthand ways to represent how atoms form covalent or ionic bonds.
Lewis Electron Dot Diagrams - Introductory Chemistry - 1st ... A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. ... With nitrogen, which has three p electrons, we put a single dot on each ...
NCl3 (Nitrogen trichloride) Lewis Structure Nitrogen trichloride (NCl3) lewis structure contains three N-Cl bonds. There is one lone pair on nitrogen atom and three lone pairs on each chlorine atom. Lewis structure of NCl3 can be drawn by using valence electrons of nitrogen and chlorine atoms. Also, there are no charges on atoms in NCl3. Steps of drawing the lewis structure of NCl3 are explained in detail in this tutorial.
What is the Lewis dot structure for nitrogen gas ... What is the Lewis dot structure for nitrogen gas? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable.
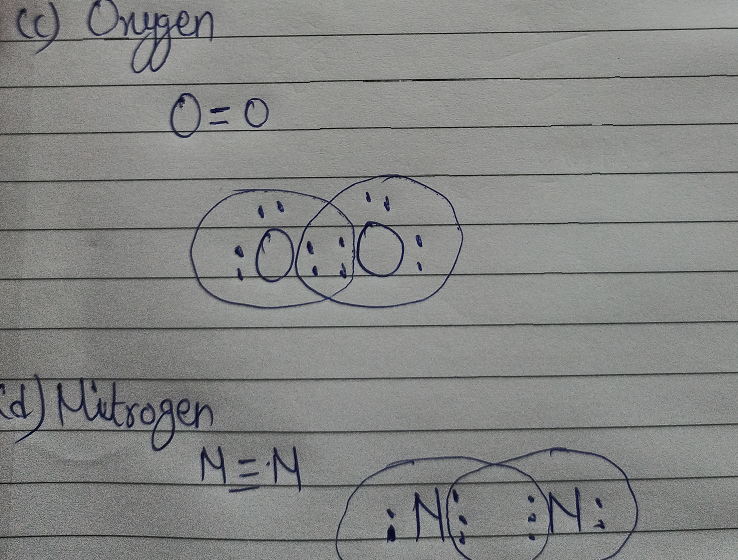








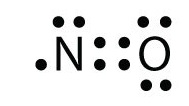
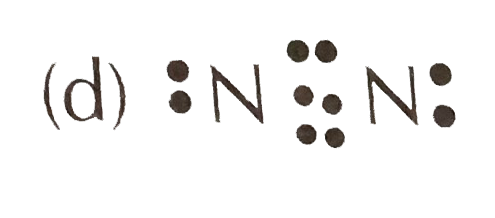


![Draw the electron dot structure of Nitrogen molecule [N = 7]](https://haygot.s3.amazonaws.com/questions/1890007_1909574_ans_16e2a124f2974b5694de1a9f3c97eebd.png)

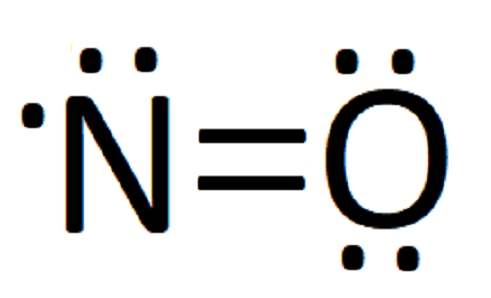













0 Response to "38 lewis dot diagram of nitrogen"
Post a Comment