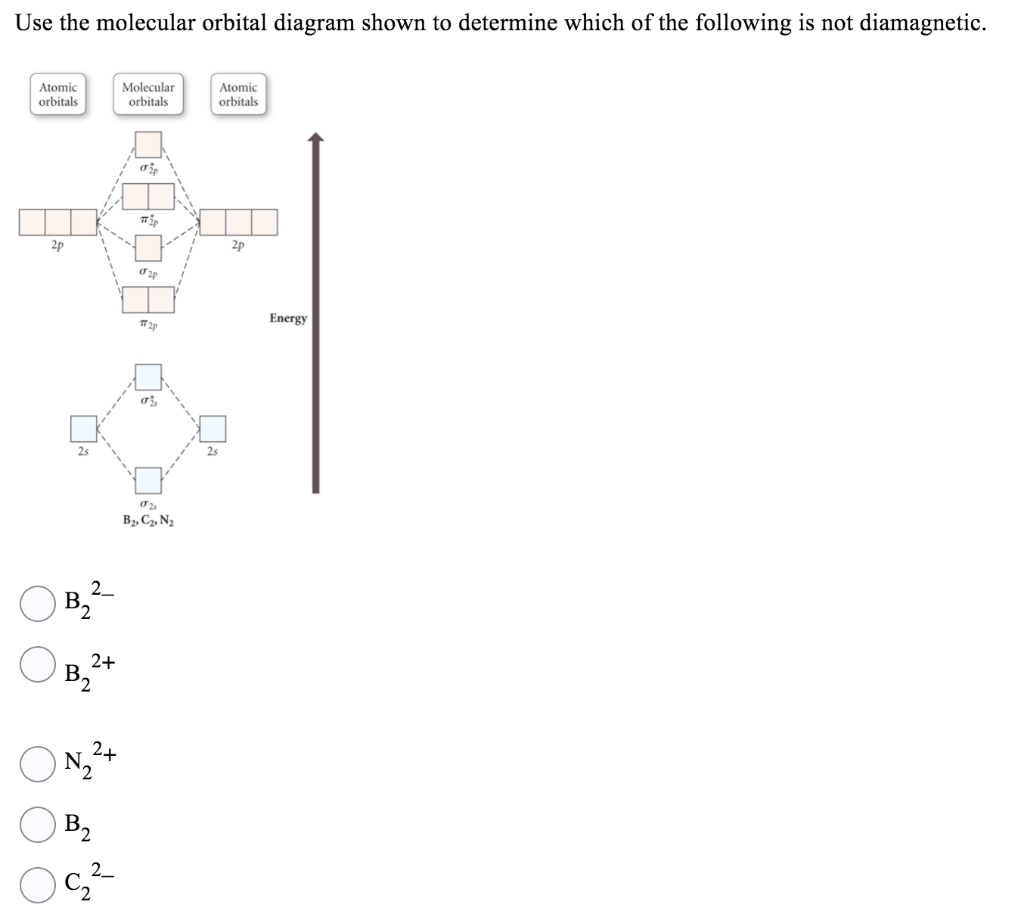
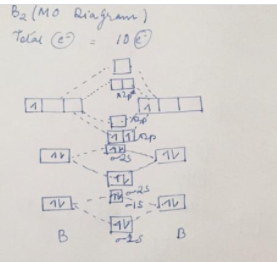
39 use the molecular orbital diagram shown to determine which of the following are paramagnetic.
Answered: Draw the molecular orbital diagram… | bartleby Answered: Draw the molecular orbital diagram… | bartleby. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+, B2, C22-, B22-, and N22+. Quiz 1 Flashcards | Quizlet 2. The bond order of a homonuclear diatomic molecule can be decreased by. removing electrons from a bonding MO or adding electrons to an antibonding MO. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) N2^2+. B) B2^2+. C) B2^2-. D) C2^2-. E) B2.
Use the molecular orbital diagram shown to... | Clutch Prep Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.a.F22+b. Ne22+c. F22-d. O22+e. F2

Use the molecular orbital diagram shown to determine which of the following are paramagnetic.
Use the molecular orbital diagram shown to... | Clutch Prep Problem: Use the molecular orbital diagram shown to determine the bond order for NO +.Is NO+ paramagnetic or diamagnetic? In molecular orbital names, s = sigma and p = pi.a. 3, paramagneticb. 2, paramagneticc. 2.5, paramagneticd. 2, diamagnetice. 3, diamagnetic chem test 3 (ch 6&7) Flashcards - Quizlet Using molecular orbital theory, determine whether F2, F2−, or (F2)^2− would have the strongest bond. F2 Using molecular orbital theory, determine the magnetism of O2 and O2−. Both O2 and O2− are paramagnetic. Formaldehyde, an organic compound frequently used to preserve biological specimens, has the formula H2CO. use orbital diagram to determine which of the following ... a. Write the molecular orbital diagram. b. Determine the bond order and state whether you expect the species to be stable or unstable. c. Determine if the species is diamagnetic or paramagnetic, and if paramagnetic, indicate the number of unpaired electrons.
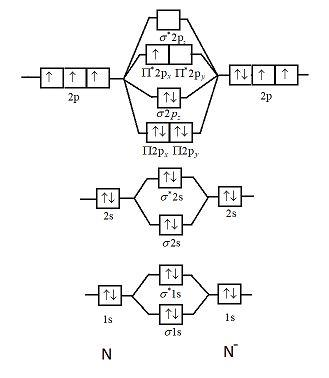
Use the molecular orbital diagram shown to determine which of the following are paramagnetic.. Use the molecular orbital diagram shown to determine which ... Use the molecular orbital diagram shown to determine which of the following is paramagnetic. Electron configuration of - Answered by a verified Tutor We use cookies to give you the best possible experience on our website. Solved Use the molecular orbital diagram shown to ... Use the molecular orbital diagram shown to determine which of the following ions is fare paramagnetic. X 2+ , Y22+ Consider that Y22+ ion has 12 total valence electrons, and X 2+ ion has 10 total valence electrons Atomic orbitals Molecular orbitals Atomic orbitals PSP 2P 2p PP 2 23 52 Select one: a. Both ions are diamagnetic b. Use the molecular orbital diagram shown to... | Clutch Prep Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A. Ne 22+ B. O 22+ C. F 22+ D. O 22- E. None of the above are paramagnetic. Learn this topic by watching MO Theory: Homonuclear Diatomic Molecules Concept Videos. Use molecular orbital diagrams to predict which of the ... asked Aug 5, 2019 in Chemistry by z1stxfile. general-chemistry. Use the molecular orbital diagram shown to determine which of the following is paramagnetic. asked Jul 15, 2019 in Chemistry by brittanyr9777. general-chemistry. Nitrogen can lose an electron to form N2+. Given the molecular orbital configuration of N2 [core] (σ2s)2 (σ *2s)2 ...
Use the molecular orbital diagram shown to determine which ... Use the molecular orbital diagram shown to determine which of the following is most stable. a. f22+ b. ne22+ c. f22- d. o22+ e. f2 Use the molecular orbital diagram shown to determine which ... Use the molecular orbital diagram shown to determine which of the following are paramagnetic. 10. Use the molecular orbital diagram shown to determine which of the following are paramagnetic Atomic orbitals Molecular orbitals Atomic orbitals pã µ 2p 2p P2p 2s /2s 2s A) B22 B) B22- C) N22 D) C22- E)... is b22+ paramagnetic or diamagnetic Paramagnetic species use molecular orbital diagram shown to determine which of the following are paramagnetic ( \ce { }! At least one unpaired electron and diamagnetic is the only paramagnetic species is not supported by inbox API. Use the molecular orbital diagram shown to determine which ... Use the molecular orbital diagram shown to determine which of the following paramagnetic. Ne_2^2+ O_2^2+ F_2^2+ O_2^2- None of the above are paramagnetic.
Solved Use the molecular orbital diagram shown to ... Chemistry questions and answers. Use the molecular orbital diagram shown to determine which of the following is paramagnetic. Atomic orbitals Molecular orbitals Atomic orbitals 2p 2P קני 25 02 02, F2 Nez F2 None of the ions are paramagnetic. 2+ 02 2-. Answered: 1 -Use the molecular orbital diagram… | bartleby 1 -Use the molecular orbital diagram shown to determine which of the following are paramagnetic a) F,2" b) Nez2* c) 0,2 d) 0,2 a O b O c O d O check_circle Expert Answer Recitation Week 10 (test 3 - Recitation 2) - GitHub Pages 2) Use molecular orbital diagrams to determine which of the following are paramagnetic. A) O2^2-B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2 Study Chapter 4 Pre-Exam Flashcards - Quizlet Use the molecular orbital diagram shown to determine which of the following is most stable. O₂²⁺ Use the molecular orbital diagram shown to determine which of the following are paramagnetic.
chemistry 1a Chapter 10 Flashcards | Quizlet Use the molecular orbital diagram shown to determine which of the following is most stable A) Ne2^2⁺ B) F2^2⁺ C) F2^2⁻ D) F2 E) O2^2⁺ E). O2^2⁺ Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A) O2 2⁺ ...
Answered: Draw a molecular orbital diagram and… | bartleby Question. Draw a molecular orbital diagram and use it to determine which of the following is paramagnetic. F 22+. Ne 22+. O 22-. O 22+. None of the above is paramagnetic. check_circle.
Use the molecular orbital diagram shown to determine which ... 55) Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A) O 2 2 ⁻ B) Ne 2 2 ⁺ C) O 2 2 ⁺ D) F 2 2 ⁺ E) None of the above are paramagnetic. Answer: D Diff: 4 Page Ref: 10.8
OneClass: Use the molecular orbital diagram shown to ... Use molecular orbital theory to describe the bonding in the following. For each one, find the bond order and decide whether it is stable. Is the substance diamagnetic or (a) Be_2 bond order Is it stable? stable unstable Is it diamagnetic or paramagnetic? diamagnetic paramagnetic (b) Ne_2 bond order Is it stable? stable unstable Is it diamagnetic or paramagnetic? diamagnetic paramagnetic
Use the molecular orbital diagram shown to... | Clutch Prep Problem: Use the molecular orbital diagram shown to determine which of the following is MOST stable.In molecular orbital names, s = sigma and p = pi.a. F2b. F22-c. O22+d. F22+e. Ne22+
Solved Use the molecular orbital diagram shown to ... We review their content and use your feedback to keep the quality high. Transcribed image text: 10. Use the molecular orbital diagram shown to determine which of the following are paramagnetic Atomic orbitals Molecular orbitals Atomic orbitals pふ 2p 2p P2p 2s /2s 2s A) B22 B) B22- C) N22 D) C22- E) B2.
CH 101, exam #4 Flashcards - Quizlet use the molecular orbital diagram shown to determine which of the following are paramagnetic. F2 ^2+ how many of the following ionic compounds are insoluble in water? NaCl KI Mg(NO3)2 CaCO3 PbI2. 2. give the theoretical yield, in moles, of CO2 from the reaction of 4.00 moles of C8H18 with 4.00 moles of O2
Use the molecular orbital diagram shown to determine ... Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.A) N22+ B) B2C) B22+D) C22-E) C22+
Use the molecular orbital diagram shown ... - Inorganic ... Use the molecular orbital diagram shown to determine which of the following is paramagnetic. Atomic orbitals Molecular orbitals Atomic orbitals की 2p 2p 27 2p os 2s 025 02, F2, Ne, F2 2+ 2+ O2 0₂ 2x 022- Nez 2+ None of the above is paramagnetic.
use orbital diagram to determine which of the following ... a. Write the molecular orbital diagram. b. Determine the bond order and state whether you expect the species to be stable or unstable. c. Determine if the species is diamagnetic or paramagnetic, and if paramagnetic, indicate the number of unpaired electrons.
chem test 3 (ch 6&7) Flashcards - Quizlet Using molecular orbital theory, determine whether F2, F2−, or (F2)^2− would have the strongest bond. F2 Using molecular orbital theory, determine the magnetism of O2 and O2−. Both O2 and O2− are paramagnetic. Formaldehyde, an organic compound frequently used to preserve biological specimens, has the formula H2CO.
Use the molecular orbital diagram shown to... | Clutch Prep Problem: Use the molecular orbital diagram shown to determine the bond order for NO +.Is NO+ paramagnetic or diamagnetic? In molecular orbital names, s = sigma and p = pi.a. 3, paramagneticb. 2, paramagneticc. 2.5, paramagneticd. 2, diamagnetice. 3, diamagnetic







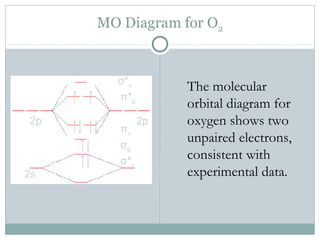

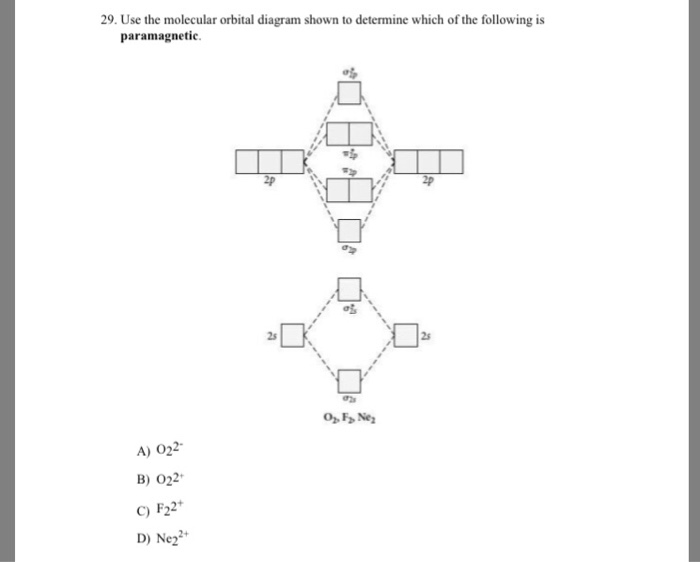

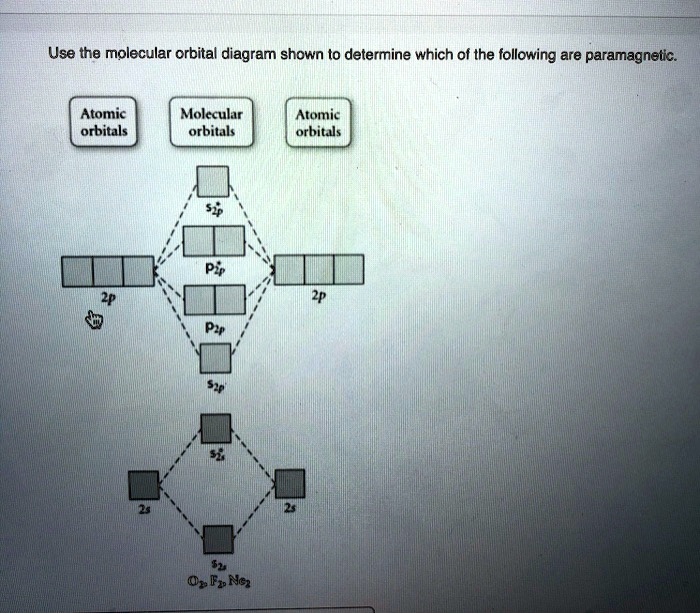
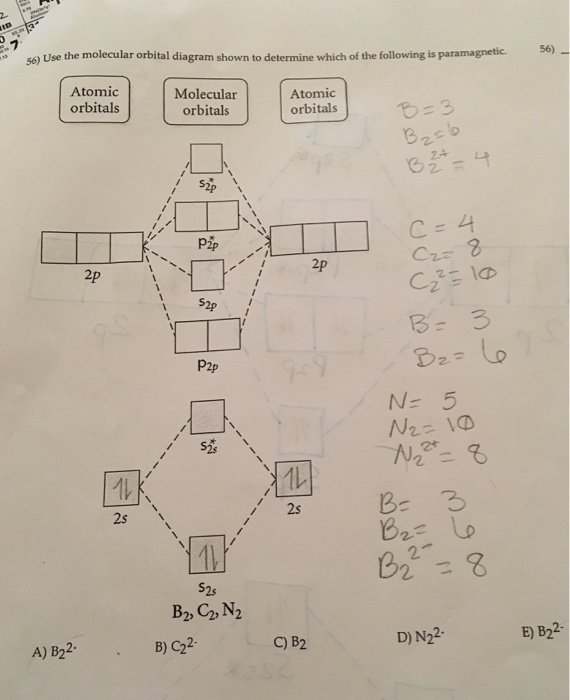
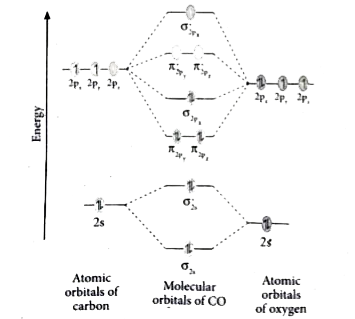

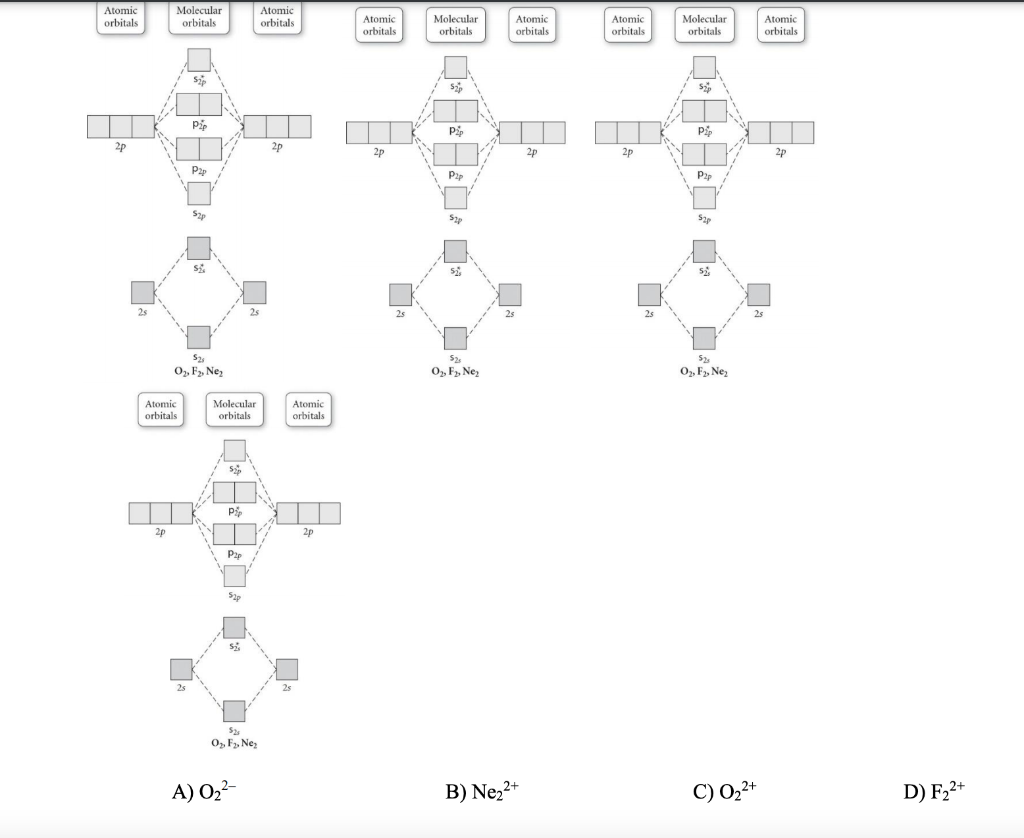


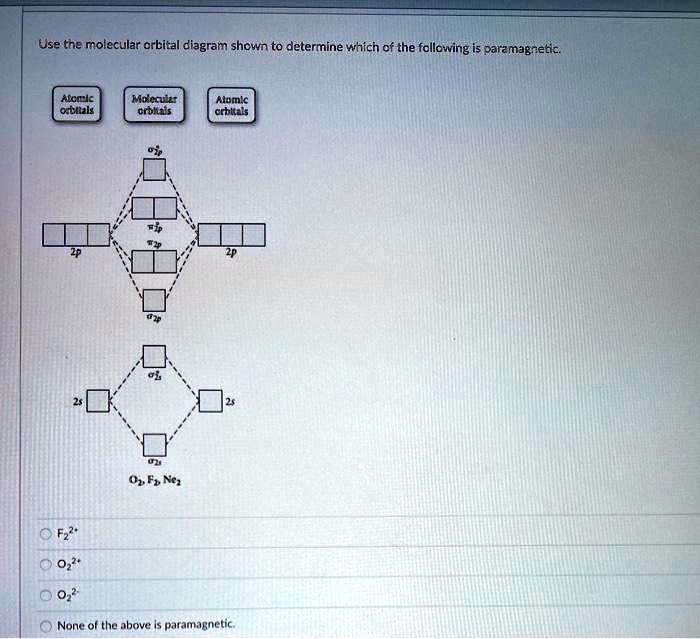


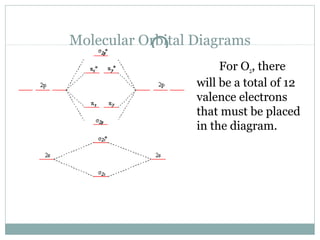

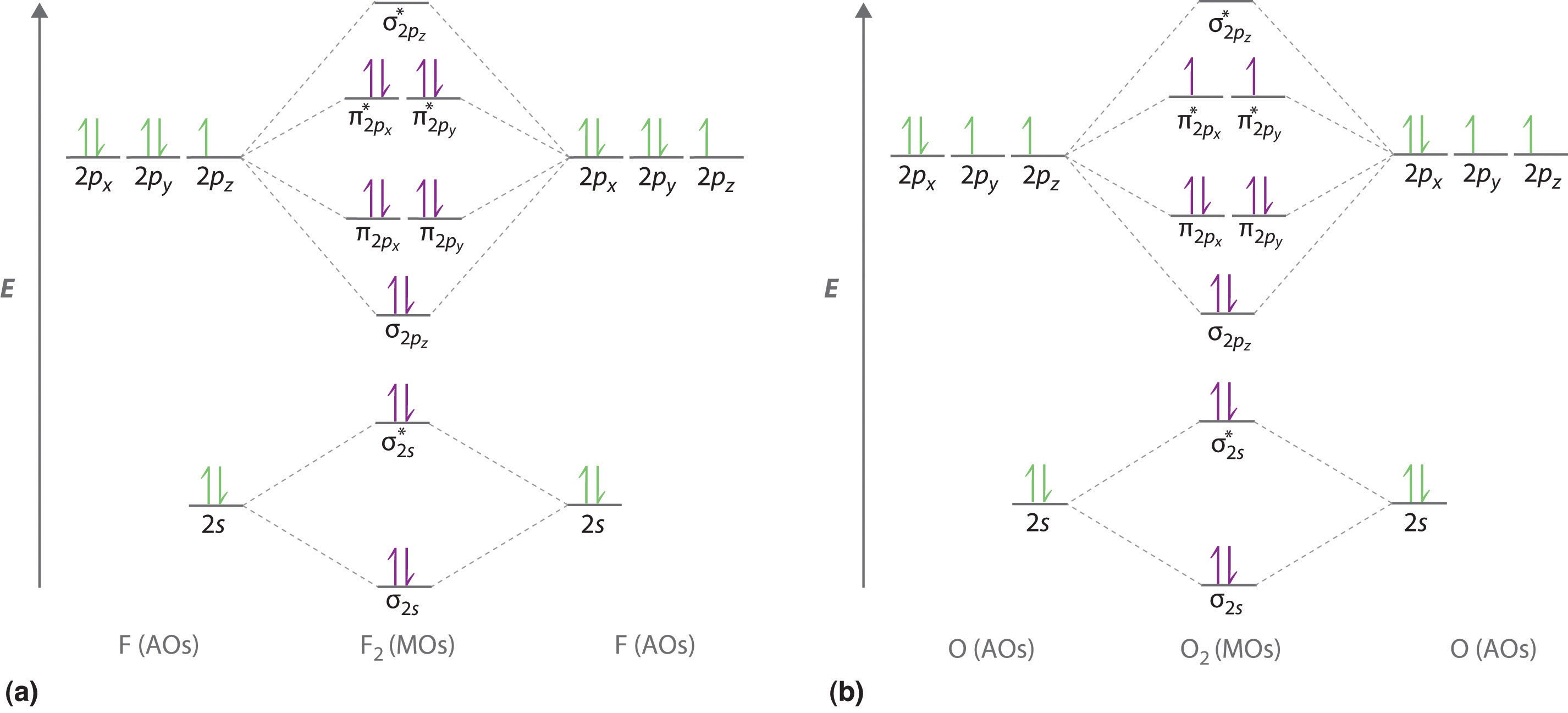
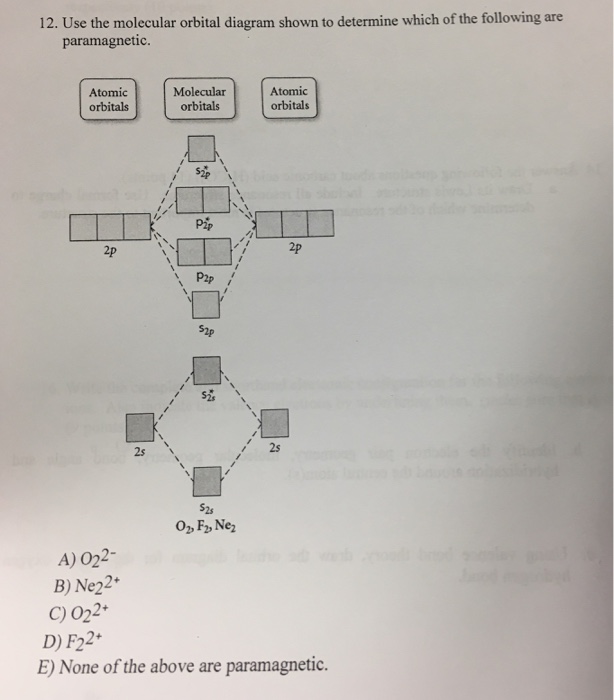
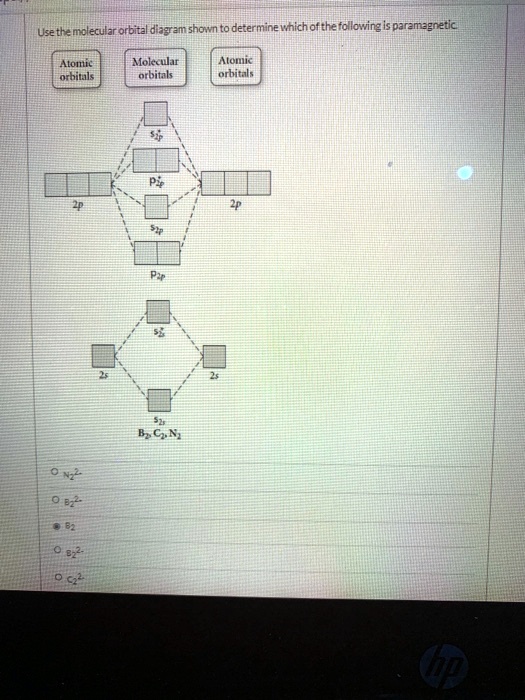


0 Response to "39 use the molecular orbital diagram shown to determine which of the following are paramagnetic."
Post a Comment