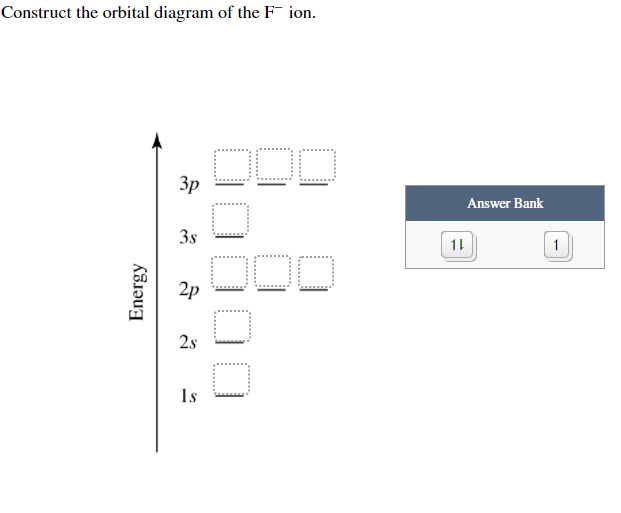
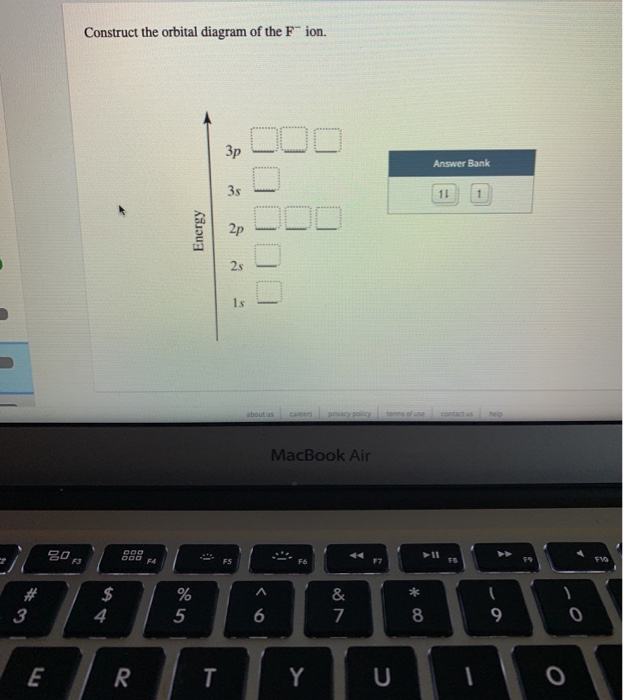
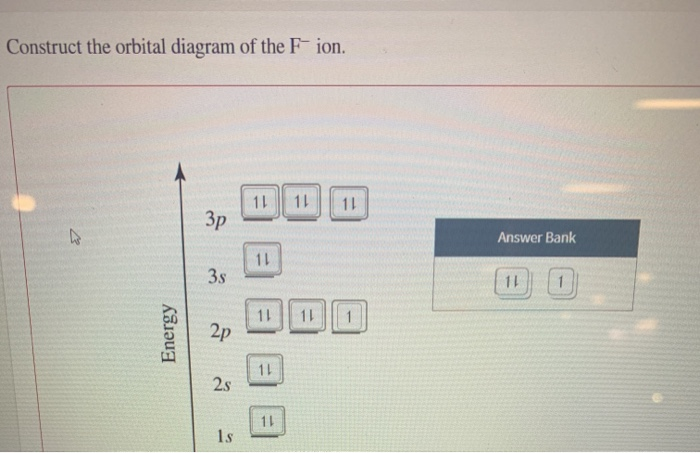
39 construct the orbital diagram of the f– ion
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau... Answer to Write the orbital diagram for Au+. Related Questions. How do you determine the atomic orbitals of an atom or molecule? How to find the isotope of. Answer to Write orbital diagram for Au+. Draw an Molecular Orbital energy diagram and predict the bond order of L 2. Using a partial orbital diagram, show . Electron Configuration
Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms.

Construct the orbital diagram of the f– ion
Construct the orbital diagram of the F^- ion. Subject: Chemistry Price: 2.85 Bought 3 Share With. Construct the orbital diagram of the F^- ion. A neutral fluorine atom has 9 electrons. Draw the molecular orbital diagram of N 2. Also find its bond order and magnetic character? Asked by Topperlearning User | 13th Jun, 2016, 02:45: PM. Expert Answer: Molecular orbital diagram of N 2 BO = [Nb-Na] = [10-4] = 3 Since all the electrons in nitrogen are paired, it is diamagnetic molecule. Figure A periodic table of partial ground- state electron configurations. Figure Write orbital diagram for Au+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons. Jul 21, · Write orbital diagram for Au+ Determine if the ion is diamagnetic or ...
Construct the orbital diagram of the f– ion. Draw the molecular orbital diagram of dioxygen and calculate bond order. Medium. View solution > Explain the molecular orbital structure, bond order, stability and magnetic behavior of Hydrogen molecule on the basis of molecular orbital theory. ... View solution > In the molecular orbital diagram for the molecular ion, N 2 + ... Fluorine(F) is the 9th element in the periodic table and its symbol is 'F'. This article gives an idea about the electron configuration of fluorine and orbital diagram, period and groups, valency and valence electrons of fluorine, bond formation, compound formation, application of different principles.Hopefully, after reading this article you will know in detail about this. Draw the molecular orbital diagram of the valence shell of a $\mathrm{F}_{2}+$ ion, and use it to determine the bond order in the ion. Answer. There are 3 more electrons in bonding orbitals than in antibonding orbitals, so the bond order is $3 / 2=1.5$ View Answer. Related Courses. Draw molecular orbital diagram for F 2 molecule. Also, gives its electronic configuration, bond order and magnetic property. Hint: The Molecular Orbital Theory (MOT) explains the formation of the molecule in a better way than Valence Bond Theory (VBT). The bond order calculations are feasible using MOT and so is the description of electronic ...
Construct the orbital diagram of the F^- ion. A neutral fluorine atom has 9 electrons. How many electrons does a F^- ion have? Question: Construct the orbital diagram of the F^- ion. A neutral fluorine atom has 9 electrons. How many electrons does a F^- ion have? This problem has been solved! See the answer Show transcribed image text Videos Orbital diagram of f-. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) F Orbital. [Kr] 4d Draw the orbital filled diagram for this ion. What is an example of a balanced chemical reaction for the formation of Cadmium hydroxide?.Jul 21, · Best Answer: orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the "boxes" with arrows, s orbital has only one box with two arrows ... Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H
Part D Construct the orbital diagram for the ion. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be filled. ANSWER: Help Reset G1 G1 G1 G1 G1 G1 G1 G1 G1 G1 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 1 s 4 d 2 s 4 f 2 p 5 ... Problem: Construct the orbital diagram of the F- ion.A neutral fluorine atom has 9 electrons. How many electrons does a F- ion have? FREE Expert Solution We are asked to construct the orbital diagram of the F - ion. F → 9 electrons Negative charge adds 1 electron more. F - → 10 electrons 97% (333 ratings) Problem Details Draw the molecular orbital diagram for N2. Label all of the atomic orbitals and molecular orbitals and put the correct number of electrons in. You do not need to draw the shapes of any of the orbitals. a) MO diagram b) Based on your MO diagram, is N2 diamagnetic or paramagnetic? c) Calculate the bond order for N2. Question: 5. Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what...
Okay, so we're gonna draw some orbital diagrams. The first one is for lithium. So you want to start by writing its electron configuration but kind of spread out of it. So it's one s. 2 And then to S one. So it? S sub level has one orbital which we represent with kind of parentheses. And then we put the two electrons in One air up and one down.
Construct the orbital diagram of the F- ion. If you can't find your institution, please check your spelling and do not use abbreviations. If your institution is not listed, please visit our Digital Product Support Community .
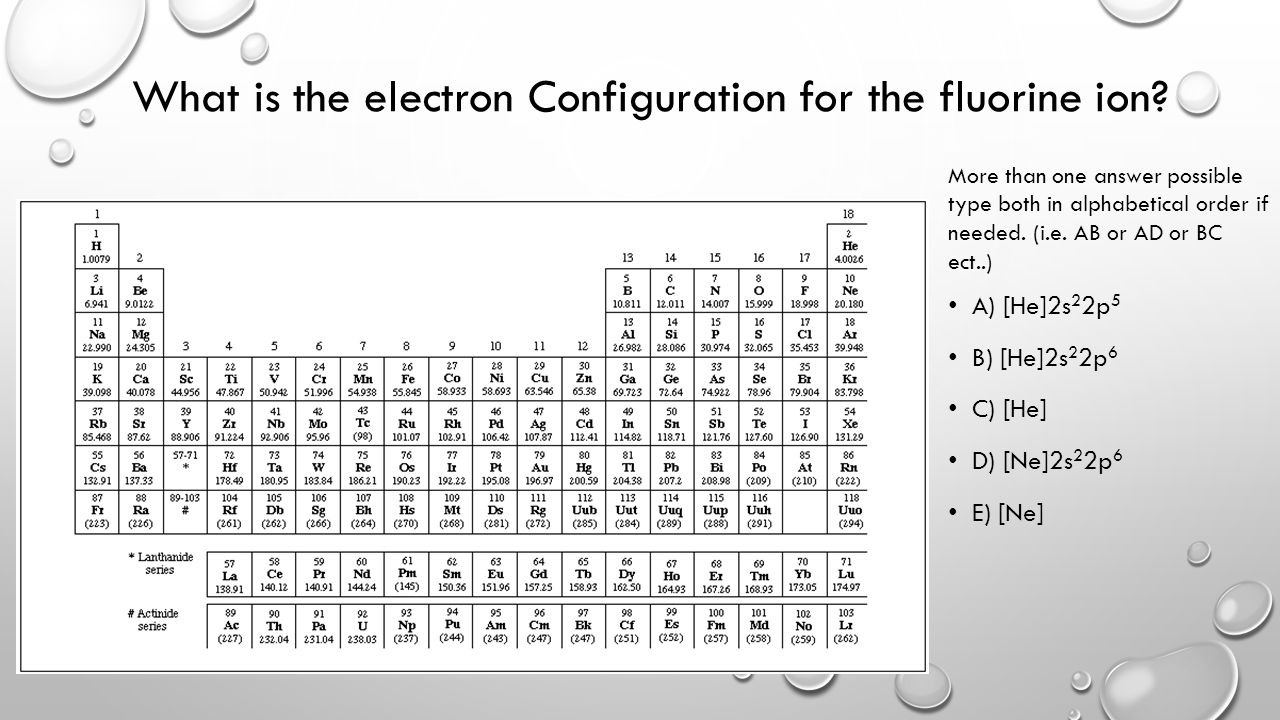
Its electron configuration will be F: 1s22s22p5 Now, the F− anion is formed when 1 electron is added to a neutral fluorine atom. Notice that the 2p-subshell of the neutral atom contains 5 electrons. Its maximum capacity is actually 6 electrons, two electrons for each p-orbital.
Construct the orbital diagram for the ion Ca2+. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Fill all group 1 targets. Not all group 2 targets will be filled. 1s 2s 2p 3s 3p 3d 4s 4p 4d 1L 1L 1L 1L 1L 1L 1L 11 1L 1s 2s 2p 3s 3p
Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11 ...
Jan 03, 2022 · The Fluoride ion formed by addition of electron to its neutral state. F + e^- rightarrow F^- Thus, F^- ion has 10 electrons. The electronic configuration of Fluoride ion is 1s^2 3s^2 2p^6. As the energy of the atomic orbital is 1s^2 < 2p^6 (2p^2_x = 2p^2_y = 2p^2_z), the orbital energy diagram is represented as shown below: Hottest videos. Widget.
This problem has been solved! See the answer. See the answer See the answer done loading. Construct the orbital diagram of the F– ion. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (22 ratings)
Molecular orbital diagram of N 2 − is shown below: This picture shows the molecular orbital diagram of N 2 − . Orbitals represented by ∗ are antibonding orbitals and the orbitals without ∗ are bonding orbitals. Bond order can be calculated by the formula: Bond order = bonding electrons - antibonding electrons 2.
Construct the orbital diagram of the {eq}F^- {/eq} ion. Orbital Diagram: Orbital diagram shows how electrons are distributed in various kinds of shells in the increasing order for a particular ion ...
We can use the d-orbital energy-level diagram in Figure \(\PageIndex{1}\) to predict electronic structures and some of the properties of transition-metal complexes. We start with the Ti 3 + ion, which contains a single d electron, and proceed across the first row of the transition metals by adding a single electron at a time.
construct construct the orbital diagram the f– ion chem 120a november 8 2005 fall 2004 8 00 – 9 20 am exam ii name prepare a molecular orbital energy level diagram for no construct a solved construct the orbital diagram the f ion a ne answer to construct the orbital diagram of the f ion a neutral fluorine atom has 9 electrons how many electrons …
Orbital Diagram Of Ti2+. Answer to Construct the orbital diagram of each atom or ion. Ti Ti2+ Ti4+. Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . When filling degenerate orbitals, electrons fill them singly first, with parallel spins is ...
Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the. Answer to Write orbital diagram for Co2+. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add.May 09, · This feature is not available right now.
Is this the correct atomic orbital diagram for a calcium (2+) ion? Please indicate "true" or "false" accordingly on your Google quiz form.
Figure A periodic table of partial ground- state electron configurations. Figure Write orbital diagram for Au+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons. Jul 21, · Write orbital diagram for Au+ Determine if the ion is diamagnetic or ...
Draw the molecular orbital diagram of N 2. Also find its bond order and magnetic character? Asked by Topperlearning User | 13th Jun, 2016, 02:45: PM. Expert Answer: Molecular orbital diagram of N 2 BO = [Nb-Na] = [10-4] = 3 Since all the electrons in nitrogen are paired, it is diamagnetic molecule.
Construct the orbital diagram of the F^- ion. Subject: Chemistry Price: 2.85 Bought 3 Share With. Construct the orbital diagram of the F^- ion. A neutral fluorine atom has 9 electrons.

















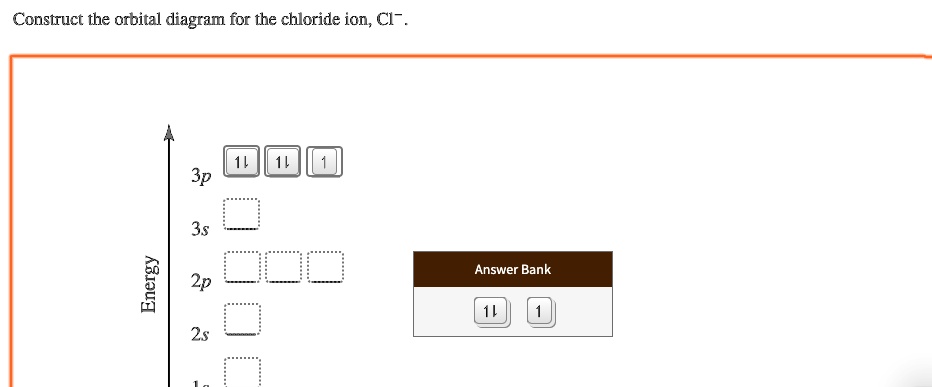




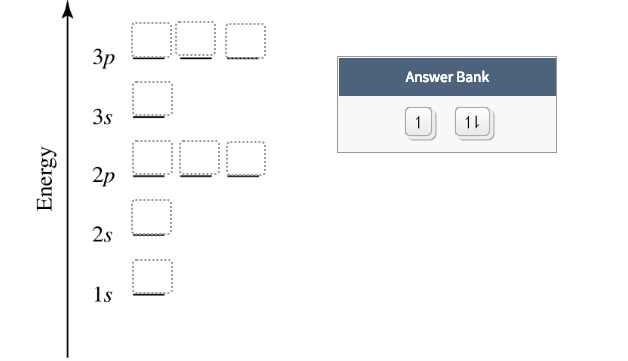

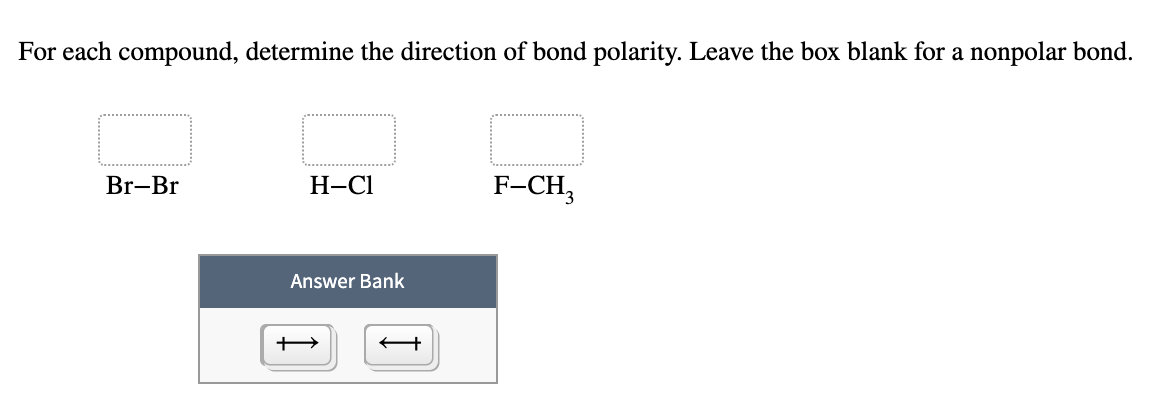

0 Response to "39 construct the orbital diagram of the f– ion"
Post a Comment