38 energy level diagram for sulfur
Energetics: 4.32 - Hess' law energy cycles and diagrams from sulfur. One route is direct (for which we don't know the energy) and the other route is via sulfur(IV) oxide. The energy change for both routes is the same. ΔH(S + O2SO2) + ΔH(SO2+ ½O2SO3) = ΔH(S + O2SO3) -297 - 98 = ΔH(S + O2SO3) ∴ ΔH for the conversion of sulfur to sulfur (VI) oxide = -395 kJ top Sulfur(S) electron configuration and orbital diagram Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit] K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2. For example, 1. n = 1 for K orbit. The electron holding capacity of K orbit is 2n2 = 2 × 12= 2 electrons. 2. For L orbit, n = 2. The electron holding capacity of the L orbit is 2n2 = 2 × 22= 8 electrons. 3. n=3 for M orbit. The maximum electron holding capacity in M orbit is 2n2 = 2 × 32 = 18 electrons. 4. n=4 for N orbit. The maximum electron holding capacity in N orbit is 2n2 = 2 × 42= 32 electrons. Therefore, the maximum electron holding capacity in the first shell...
Energy Levels of Neutral Sulfur ( S I ) - NIST Energy Levels of Neutral Sulfur ( S I ) Configuration Term J Level(cm-1) Ref. 3s23p4 3P 2 0.000 MZM90 1 396.055 MZM90 0 573.640 MZM90 3s23p4 1D 2 9238.609 MZM90 3s23p4 1S 0 22179.954 MZM90 3s23p3(4S°)4s 5S° 2 52623.640 MZM90 3s23p3(4S°)4s 3S° 1 55330.811 MZM90 3s23p3(4S°)4p 5P 1 63446.065 MZM90 2 63457.142 MZM90 3 63475.051 MZM90 3s23p3(4S°)4p 3P 1

Energy level diagram for sulfur
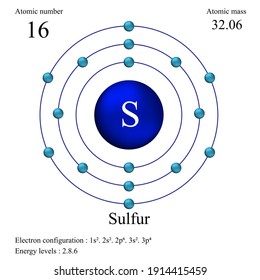
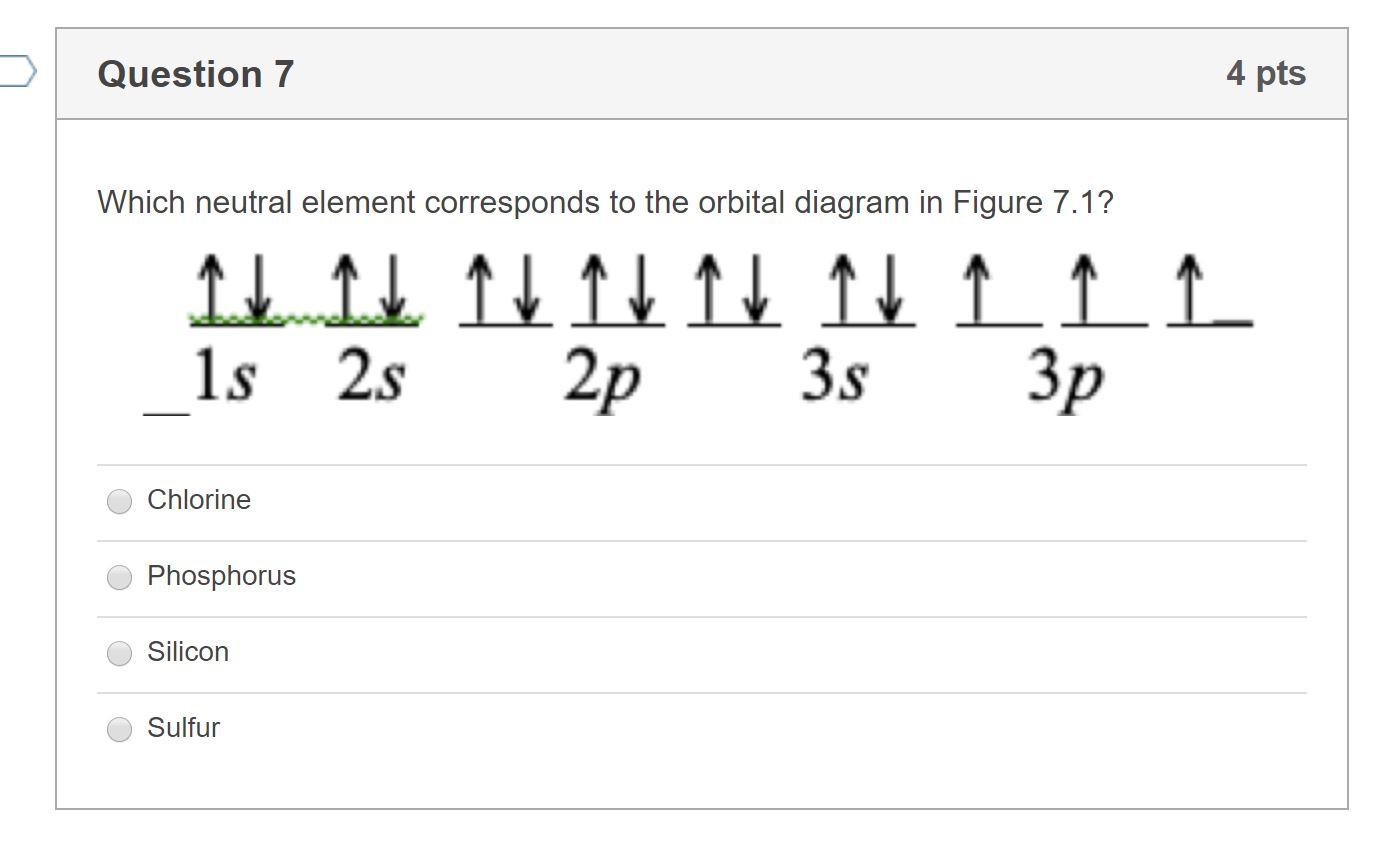
Solved 1. In the first energy diagram, correctly place the ... In the first energy diagram, correctly place the electrons for elemental sulfur in the appropriate energy levels/orbitals. Sulfur Orbital diagram, Electron configuration, and ... Sulfur is situated in Group 16th or 6A and has an atomic number of 16. The first shell of Sulfur has 2 electrons and the outer shell or valence shell of Sulfur has 6 electrons, hence, the number of valence electrons in the Sulfur atom is 6. The orbital diagram for Sulfur is drawn by following three principles - the Aufbau principle, Hund's ... Question Video: Deducing the Energy Level Diagram ... Therefore, a sulfur atom will have a total of 16 electrons. Energy level K will hold the first two electrons. The next energy level L will hold the next eight electrons in the sulfur atom. With only six electrons remaining, they will all be found in energy level M.
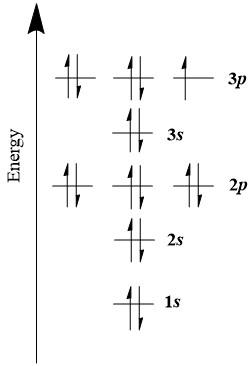
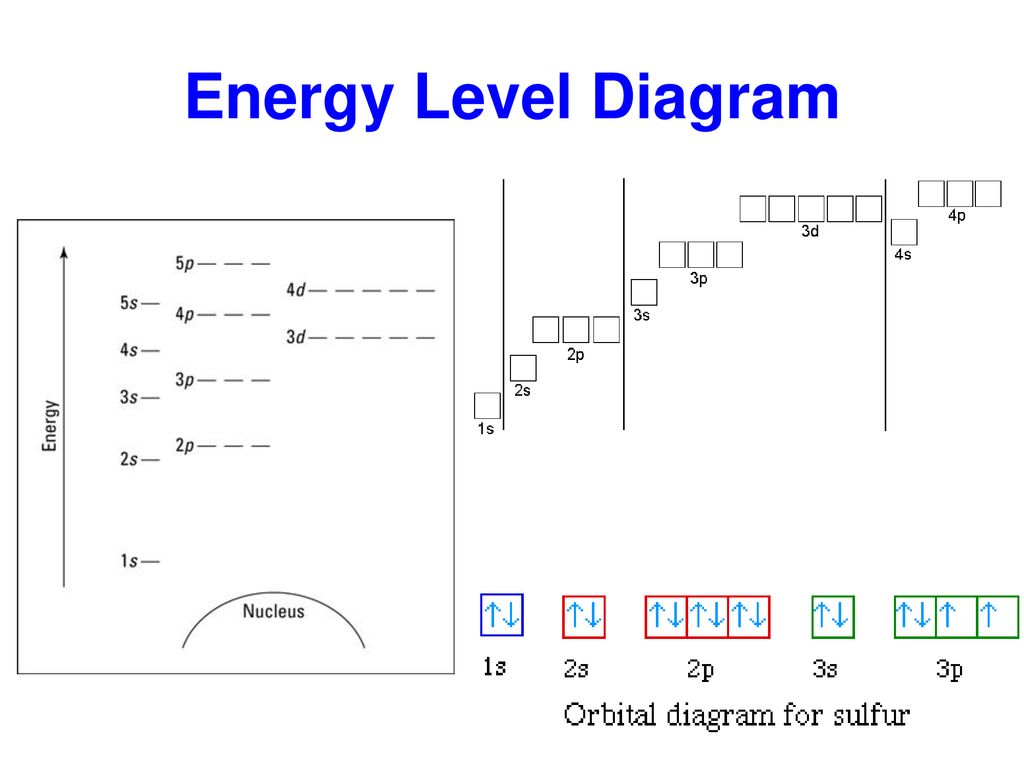
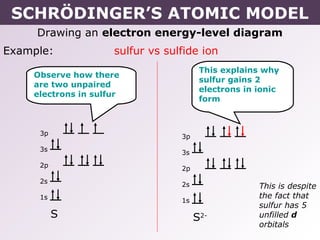
Energy level diagram for sulfur. Orbital Filling Diagram For Sulfur The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. Solved 8. Sulfur gains two electrons to be a sulfide (S2 ... Sulfur gains two electrons to be a sulfide (S2) anion. (a) Draw an energy level diagram of the sulfide ion according to the trends you learned in class. [24] Page 3 of 4 40 March 2021 (b) Provide the full electron configuration of the sulfide ion. [1A] (c) Provide the condensed electron configuration of the sulfide ion. PDF 03 0620 32 2RP - GCE Guide (b) Name one source of sulfur..... [1] (c) When carbon is heated with sulfur, carbon disulfide, CS 2, is produced. C + 2S → CS 2 (i) Complete the energy level diagram for the production of carbon disulfideby writing these formulae on the diagram: C + 2S CS 2. energy progress of reaction [1] PhysicsLAB: Energy-Level Diagrams PhysicsLAB: Energy-Level Diagrams. Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another. These energies differences correspond to the wavelengths of light in the discreet spectral lines emitted by an atom as it goes through de-excitation or by the ...
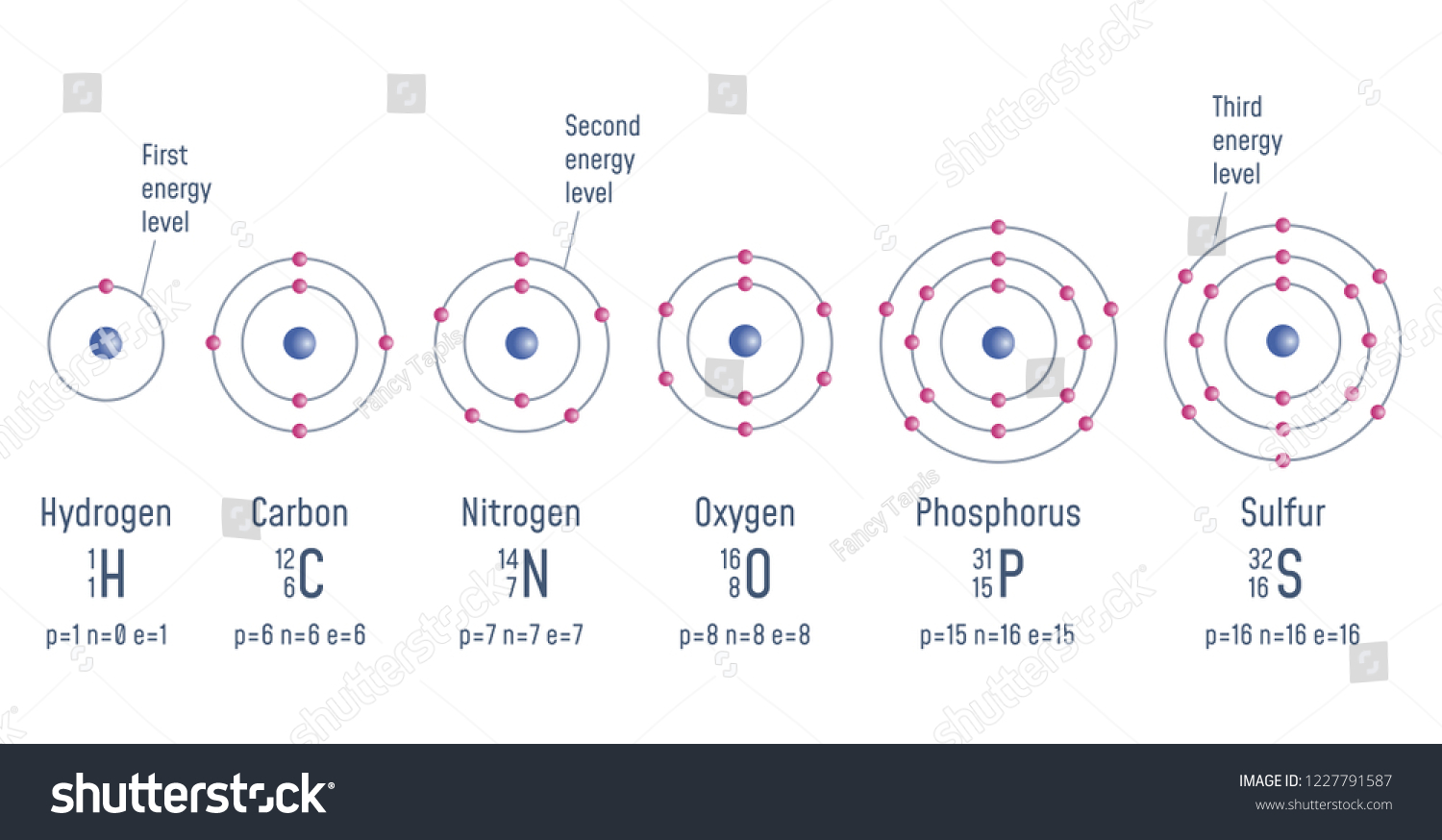
Energy Level of an Atom - Energy State and Energy level ... The first four energy levels are shown here. The first energy level is also called level 'K'. The second level is called level L, third energy level as M, and so on. The electrons from energy level K contains the least energy whereas the levels that are far from the nucleus contains more energy What is the orbital diagram for sulfur? - FindAnyAnswer.com Sulfur atoms have 16 electrons and the shell structure is 2.8. 6. The ground state electron configuration of ground state gaseous neutral sulfur is [Ne]. 3s2. Similarly, you may ask, what is the orbital diagram for silicon? Silicon has 14 electrons in the following orbital configuration 1s2 2s2 2p6 3s2 3p2 when neutral in charge. Show The Orbital Filling Diagram For Sulfur The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. Structure and energy diagram of MoS2 monolayer sample. a ... Download scientific diagram | Structure and energy diagram of MoS2 monolayer sample. a Illustration of MoS2 lattice structure, with a sulfur monovacancy; top view, side view. Theoretical work has ...
Bohr Diagram For Sulfur The bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom. for potassium, you would put 2 electrons on the first layer, 8 on the second layer, and 9 on the third layer. This is because the atomic number of Potassium (K) is 19, therefore has 19 protons and 19 electrons. How to Make a Model of a Sulfur Atom. CHEM 101 - Lecture 5 - Gonzaga University Let us use our orbital energy level diagram to predict the electron configuration of sulfur. The atomic number of sulfur is 16, so there will be 16 electrons in a neutral sulfur atom. We start filling in electrons in our diagram (refer to the figure below, panel (i)) according to the aufbau principle, starting with the lowest energy orbital. Types of Bonds and Orbitals - Atomic Orbitals and ... - Shmoop For sulfur this would be 16. We can also tell from this notation and from the energy level diagram that sulfur needs 2 more electrons to complete its outer shell. Use the following table to help remember how many subshells, orbitals, and total electrons there can be in each energy level (shell). Sulfur (S) - Chemical Elements.com Number of Energy Levels: 3 First Energy Level: 2 Second Energy Level: 8 Third Energy Level: 6 Isotopes. Isotope: Half Life: S-32: Stable: S-33: Stable: S-34: Stable: S-35: 87.2 days: S-36: Stable: Facts Date of Discovery: Known to the ancients Discoverer: Unknown Name Origin: From the Latin word sulfur (brimstone) Uses: matches, gunpowder ...
Sulfur Bohr Model - How to draw Bohr diagram for Sulfur (S ... Here, we will draw the Bohr diagram of the Sulfur atom with some simple steps. Steps to draw the Bohr Model of Sulfur atom 1. Find the number of protons, electrons, and neutrons in the Sulfur atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei.
[Solved] Draw enthalpy profile diagrams for: a. The ... Answer to Draw enthalpy profile diagrams for: a. The combustion of sulfur to form sulfur dioxide b. The endothermic reaction H 2 O(g) + C(s) → H 2 (g) + CO(g) | SolutionInn ... Draw a reaction-energy diagram for a two-step endothermic reaction with a rate-limiting ... The IBM System/370 architecture uses a two-level memory structure and ...
Electron Configuration for Sulfur (S) - UMD In order to write the Sulfur electron configuration we first need to know the number of electrons for the S atom (there are 16 electrons). When we write the configuration we'll put all 16 electrons in orbitals around the nucleus of the Sulfur atom. In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital.
PDF Energy Levels of Sulfur, S I Through S XVI ENERGY LEVELS OF SULFUR, S I THROUGH S XVI SI 823 Z=16 Ionization energy 83559.1 ± 1.0 cm-1 (10.36001 ± 0.00012 eV) Levels Below the Principal Ionization Limit A large extension of the analysis of this spectrum was given in the 1933 papers by Frerichs, by Ruedy, and by Meissner et al. No more recent observations are avail
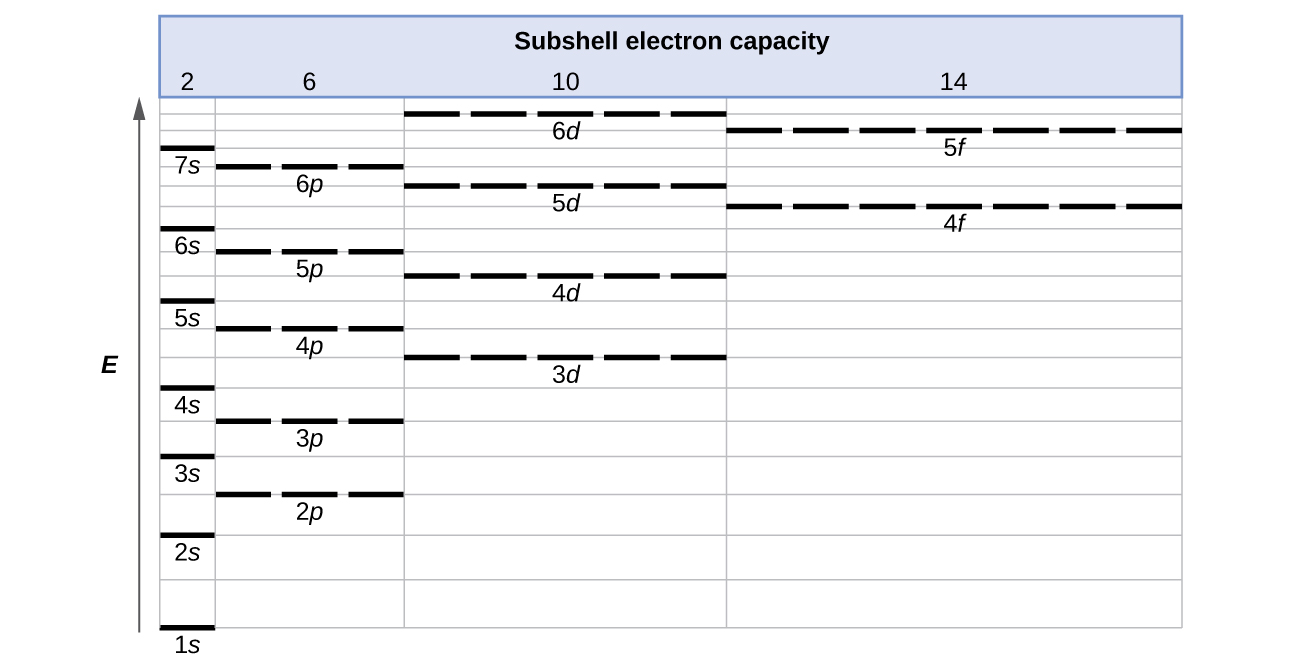
Energy Level Diagram - Different Energy Shells Around the ... Below is a blank energy level diagram which helps you depict electrons for any specific atom. At energy level 2, there are both s and p orbitals. The 2s has lower energy when compared to 2p. The three dashes in 2p subshells represent the same energy. 4s has lower energy when compared to 3d. Therefore, the order of energy level is as follows: s ...
Bohr Diagram For Sulfur - schematron.org Jul 04, 2019 · The sulfur atom has 16 protons, 16 neutrons and 16 electrons in three different energy levels, or orbits. Physics suggests that electrons do not. Here we go: 1st energy level - 2 electrons max 2nd energy level - 8 electrons max 3rd+ levels - 18 electrons max. Number of Protons/Electrons: Number of Neutrons: Classification: Non- metal.
Energy level diagram for Molecular orbitals - Chemical ... Energy level diagram for Molecular orbitals The first ten molecular orbitals may be arranged in order of energy as follow: σ (1s) <σ∗(1s) < σ (2s) <σ∗(2s) < π (2px) = π (2py) < σ (2pz) < π∗(2px) =π∗(2py) <π∗( 2pz) Relationship between electronic configuration and Molecular behaviour
What Is the Orbital Diagram for Sulfur? - Reference.com Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4.An orbital diagram illustrates how the electrons pair off in each orbital. Electrons are first placed in lower energy levels before the higher energy levels.
Hybridization of Atomic Orbitals - University of Illinois ... The energy diagram for carbon in CO 2 is shown below. What is the hybridization of oxygen in CO 2. Each oxygen has two lone pairs and forms one s bond and one p bond. This means that there must be three hybridized orbitals and one unhybridized p orbital to make the p bond. This is sp 2 hybridization.
Bohr Diagram Of Sulfur - schematron.org Sep 30, 2018 · Sulfur Bohr Model Diagram. Sulfur at Chemical schematron.org Basic Information | Atomic Basic Information. Name: Sulfur Symbol: S [Bohr Model of Sulfur], Number of Energy Levels: 3. In the Bohr model, electrons are confined to concentric spheres around the nucleus numbered as n=1, 2, 3,. The sphere n = 1 can accommodate two, the n = Model ...
Bohr Diagram Of Sulfur - wiringall.com Sulfur Bohr Model Diagram. See all . All About Sulfur! Bohr Diagram. Picture. Powered by Create your own unique website with customizable templates. Get Started.A Bohr Atom Model consists of a CORE of 16 Protons and 16 Neutrons (32 total) AND 16 total Electrons in the Energy Levels, the inner-most level has 2, the second level has 8, and the ...
Question Video: Deducing the Energy Level Diagram ... Therefore, a sulfur atom will have a total of 16 electrons. Energy level K will hold the first two electrons. The next energy level L will hold the next eight electrons in the sulfur atom. With only six electrons remaining, they will all be found in energy level M.
Sulfur Orbital diagram, Electron configuration, and ... Sulfur is situated in Group 16th or 6A and has an atomic number of 16. The first shell of Sulfur has 2 electrons and the outer shell or valence shell of Sulfur has 6 electrons, hence, the number of valence electrons in the Sulfur atom is 6. The orbital diagram for Sulfur is drawn by following three principles - the Aufbau principle, Hund's ...
Solved 1. In the first energy diagram, correctly place the ... In the first energy diagram, correctly place the electrons for elemental sulfur in the appropriate energy levels/orbitals.


















0 Response to "38 energy level diagram for sulfur"
Post a Comment