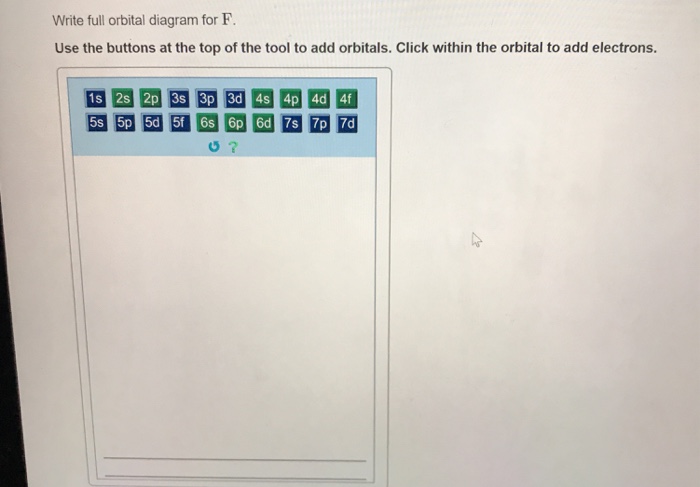
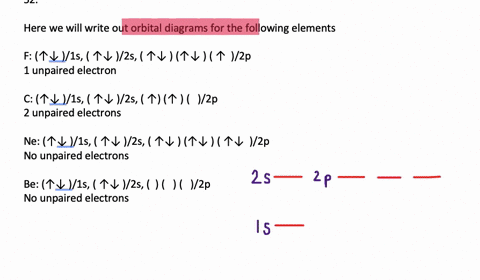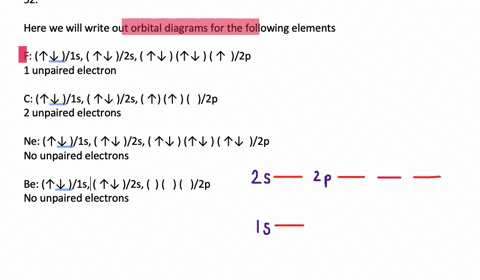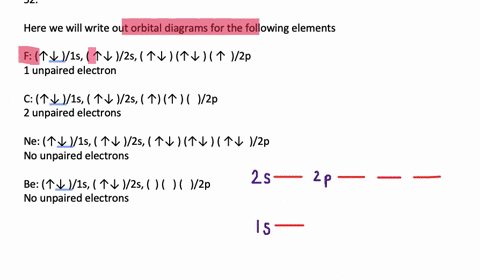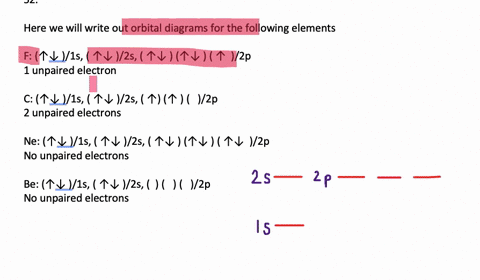
41 write full orbital diagram for f.
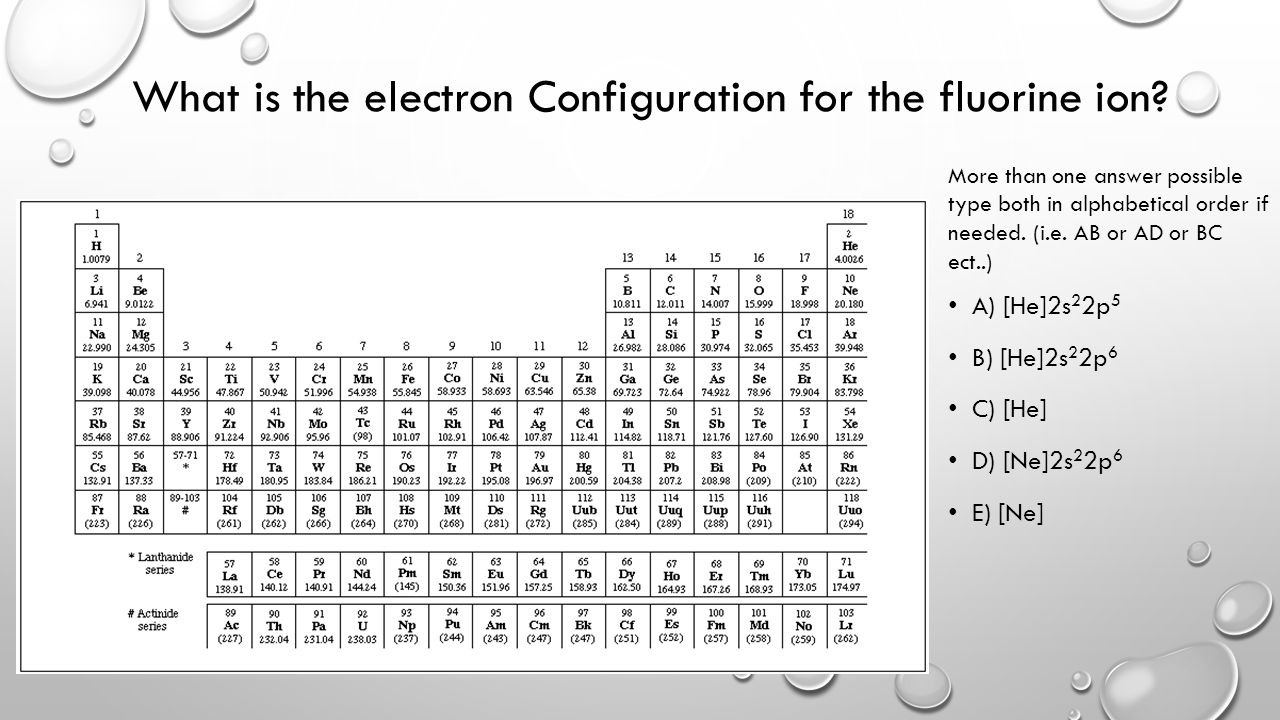
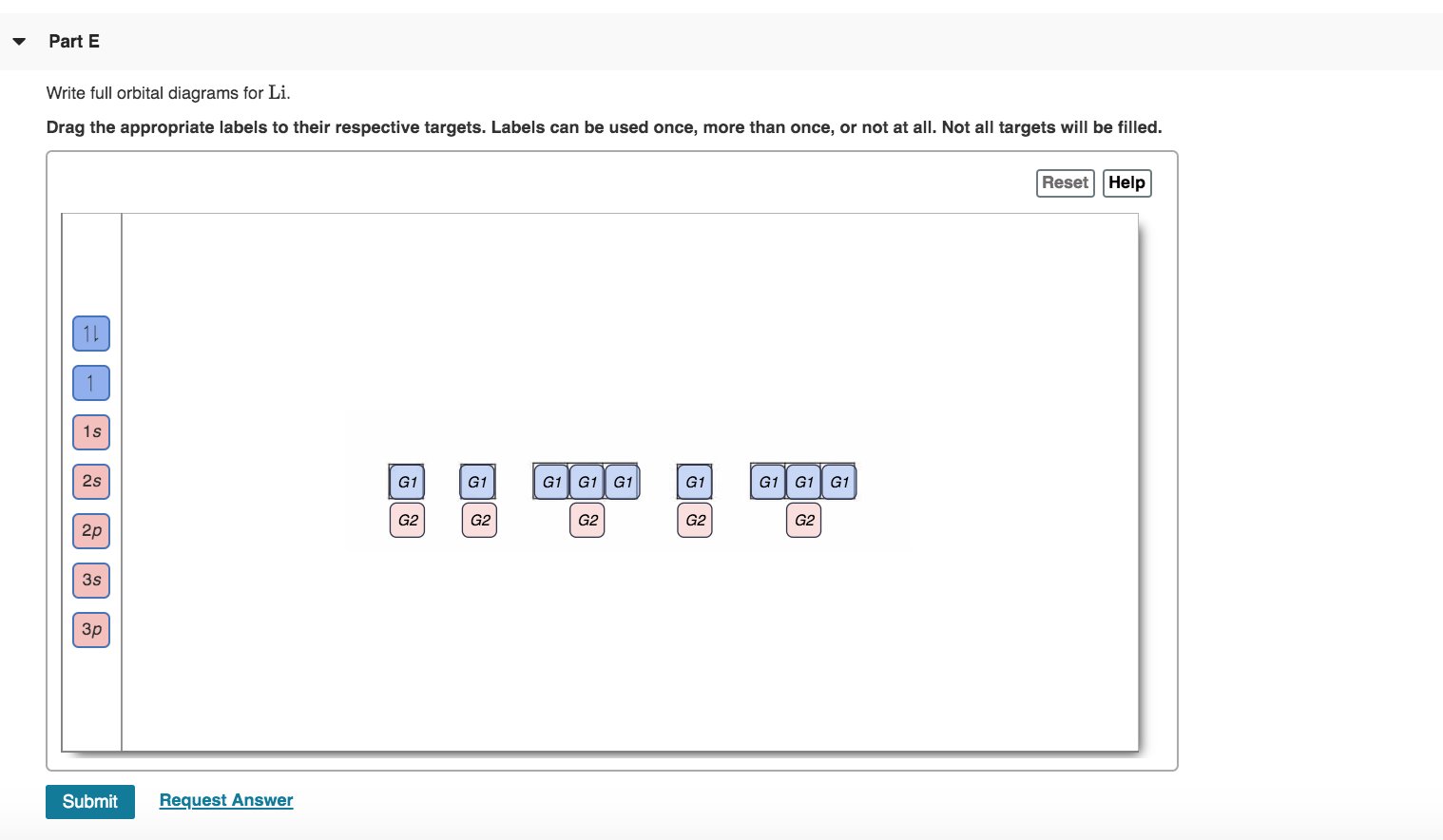
Jan 01, 2022 · Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ... 23 Jun 2016 — In your case, you must find the electron configuration of the fluoride anion, F− , so start by writing the electron configuration of a ...1 answer · F−:1s22s22p6 Explanation: A good starting point for when you must find the electron configuration of an ion is the electron configuration of the ...
Hey there! We have to write orbital diagram to represent the electron configurations without hybridization for F in SF2. To solve this problem, we will need to write the electron configuration for fluorine (F) atom. Fluorine belongs to group 7A. 81% (108 ratings) Sign up for free to view this solution. Sign up for free.

Write full orbital diagram for f.
Answer to: (a) Write the full orbital diagram for F. (b) Indicate the number of unpaired electrons in it. By signing up, you'll get thousands of... Find step-by-step Chemistry solutions and your answer to the following textbook question: Write the full orbital diagram for each element. a. N, b. F, c.1 answer · Top answer: In writing an orbital diagram the subshells determine the number of orbitals, where s-subshell has 1 orbital, p-subshell has 3 orbitals, d-subshell ... The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Orbital diagrams use the same basic format, but instead of numbers for the electrons, they ...
Write full orbital diagram for f.. Solution for Write the full orbital diagram for element. F This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... Fluorine(F) electron configuration with full orbital diagram Fluorine(F) is the 9th element in the periodic table and the first element in group-17. The standard atomic mass of fluorine is 18.998403 and its symbol is 'F'. The g-orbital block comes after the f-orbital block and consists of elements that have not been synthesized yet. Provide the full set of 3 quantum numbers (n, l, …
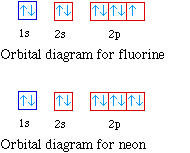
Transcribed image text: Part A Write the full orbital diagram for F. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Reset Help 11 1s G1 G1 G1 G1 G1 G2 G2 G2 2s 2p Write the full orbital diagram for C. Drag the appropriate labels to their respective targets. An orbital diagram is a different way to show the electron configuration of an atom. It symbolizes the electron as an arrow in a box that represents the orbital. The orbital for a hydrogen atom: (is a box with 1s under it and an H next to it. It has one arrow in it facing up) Neon (Ne) electron configuration with full orbital diagram. Neon (Ne) is the tenth element in the periodic table and the 2nd element in group-18. The atomic number of neon is 10 and its symbol is 'Ne'. The standard atomic mass of neon is 20.1797 and it is an inert element. 8. For an atom of 9F, write its: Full electron configuration: 1s? Draw a full orbital diagram (es as arrows in boxes with labels): Count the unpaired e s = 9F atom is diamagnetic ? paramagnetic (mark one) Write four quantum numbers n, I, m, m, separately for each of the valence e s in the configuration:
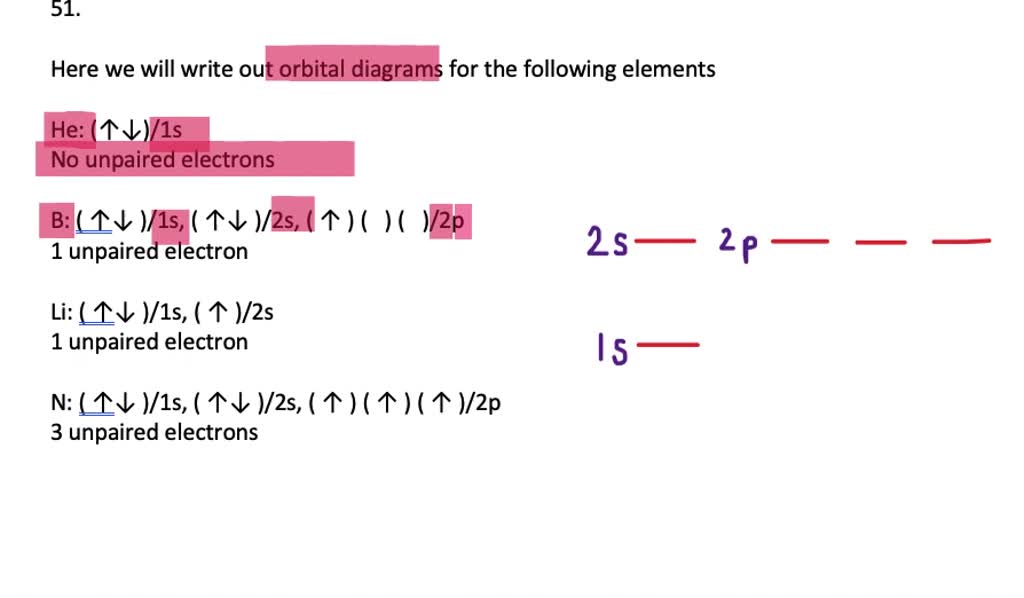
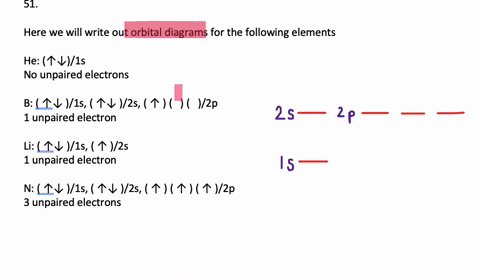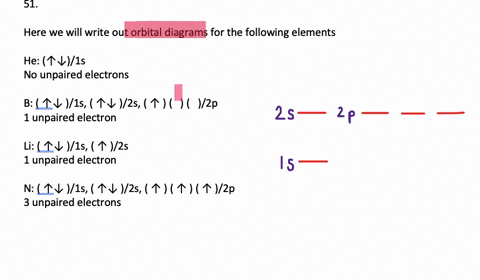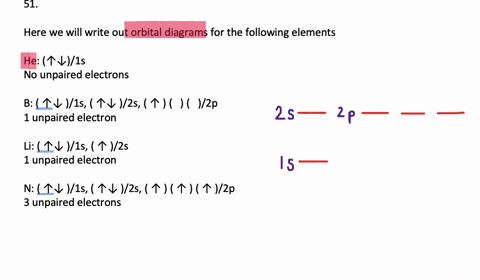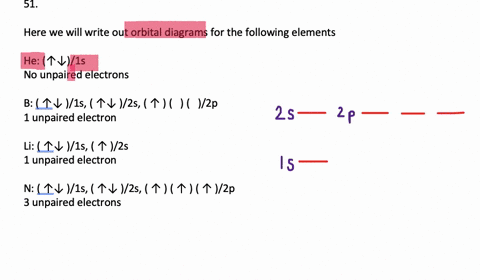
The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ... Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Where are the Electrons? Write the full electron configuration, short-hand electron configuration, and fill in the orbital diagrams, for the following elements. 1. An orbital diagram is similar What is the orbital diagram for. For example, write the electron configuration of scandium, Sc: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. So for scandium the 1 st and 2 nd electron must be in 1s orbital, the 3 rd and 4 th in the 2s, the 5 th through 10 th in the 2p orbitals, etc. 6/14/ Ch 8 4/18 Correct Part B Complete ... The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is "1s"^2"2s"^2"2p"^2". The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with ...
The 3dx² - y² orbital looks exactly like the first group, except that that the lobes are pointing along the x and y axes, not between them. The 3dz² looks like a p orbital wearing a doughnut around its waist. f ORBITALS. At the fourth and higher levels, there are seven f orbitals in addition to the 4s, 4p, and 4d orbitals.
For Strontium:a) Write the full electron configuration.b) Write the condensed electron configuration.c) Predict the common ion for Strontium.d) Write the con...
Correct Part F Indicate the number of unpaired electrons in it. Express your answer as an integer. ANSWER: ANSWER: = 0 Correct Part G Write full orbital diagram for. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons.
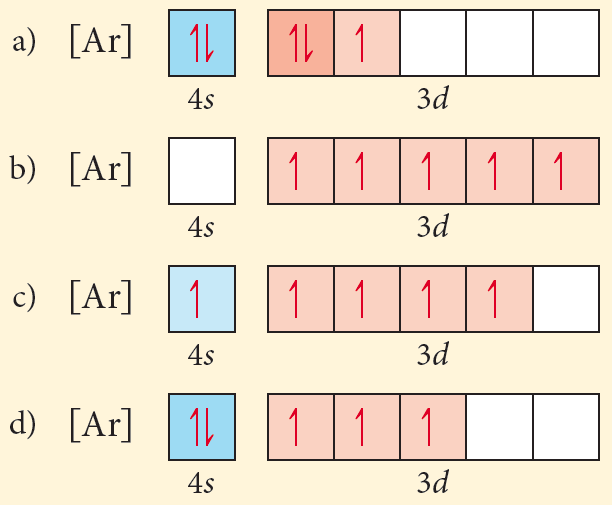
Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Part C Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element.
Chemistry questions and answers. Part A Write full orbital diagram for F. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Reset Help @@@BE G1G1GT Submit Request Answer - Part B Indicate the number of unpaired electrons in F. Express your answer as an integer.
Fluorine is the ninth element with a total of 9 electrons. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital. The remaining five electrons will go in the 2p orbital. Therefore the F electron configuration will be 1s 2 2s 2 2p 5.
Sodium(Na) electron configuration with full orbital diagram. Sodium is the eleventh element in the periodic table and the 3rd element in group-1. The atomic number of sodium is 11 and its symbol is ‘Na’. The standard atomic mass of sodium is 22.989769. The period of sodium is 3 and it is a s-block element. This article gives an idea about the electron configuration of sodium(Ne) …
Q. Write orbital diagrams for each of these ions.V5+ Q. Enter the orbital diagram for the ion Zr2 +. Q. Write orbital diagram to represent the electron configurations-without hybridization-for F in SF2.
Use this tool to draw the orbital diagram. 3d. 4p. Draw orbital diagrams for the following elements. Write the electron configuration (full, and in core notation). 1. scandium. ↑↓. ↑↓. ↑↓ ↑↓ ↑↓. ↑↓. Contrary to what you may have seen, for Sc and the remaining elements, the 4s is not lower in energy than the 3d. In fact ...
In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two ...
How to Write an Electron Configuration. The symbols used for writing the electron configuration start with the shell number (n) followed by the type of orbital and finally the superscript indicates how many electrons are in the orbital. For example: Looking at the periodic table, you can see that Oxygen has 8 electrons.
Write the orbital diagram for sulfur and determine its number of unpaired electrons. Electron configuration: 1s2 2s2 2p6 3s2 3p4. Orbital diagram: 1s= 1 up 1 down. 2s= 1 up 1 down. 2p= 1 up 1 down 1 up 1 down 1 up 1 down. 3s= 1 up 1 down. 3p= 1 up 1 down 1 up 1 up. ... Write the full orbital diagram for each element. A.) N B.) F C.) Mg D.) Al.
Write full orbital diagrams and indicate the number of unpaired electrons fo… 04:14. Write the full orbital diagram for each element. a. N b. F c… 02:16. Write the full orbital diagram for each element. \begin{equation} \beg… 03:30. Draw orbital-filling diagrams for the following atoms. ...
Write the full electron configuration, short-hand electron configuration, and fill in the orbital diagrams, for the following elements. 1. Nitrogen 1s 2s 2p 3s 2. Chlorine 1s 2s 2p 3s 3p 3. Sodium 1s 2s 2p 3s 3p 4. Neon 1s 2s 2p 3s 3p 5. Nickel 1s 2s 2p 3s 3p 4s 3d 6) Vanadium 1s 2s 2p 3s 3p 4s 3d 7) Copper 1s 2s 2p 3s 3p 4s 3d
The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Orbital diagrams use the same basic format, but instead of numbers for the electrons, they ...
Find step-by-step Chemistry solutions and your answer to the following textbook question: Write the full orbital diagram for each element. a. N, b. F, c.1 answer · Top answer: In writing an orbital diagram the subshells determine the number of orbitals, where s-subshell has 1 orbital, p-subshell has 3 orbitals, d-subshell ...
Answer to: (a) Write the full orbital diagram for F. (b) Indicate the number of unpaired electrons in it. By signing up, you'll get thousands of...


![Electron Configuration | Chemistry [Master]](https://s3-us-west-2.amazonaws.com/courses-images/wp-content/uploads/sites/1941/2017/05/30162457/cg10c3-010.png)

















/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)






0 Response to "41 write full orbital diagram for f."
Post a Comment