41 potassium electron dot diagram
How many valence electrons are in potassium and what does a dot diagram of it look like? Just from $13/Page. Order Essay. The following diagram illustrates the electron dot diagram for potassium. Notice that the single dot representing the valence electron can be written on any of the four sides of the symbol. potassium K+ 419 Na+ 3 ... Complete the dot-and-cross diagram to show the electron arrangement in a molecule of chlorine. Show the outer shell electrons only. [2] 6
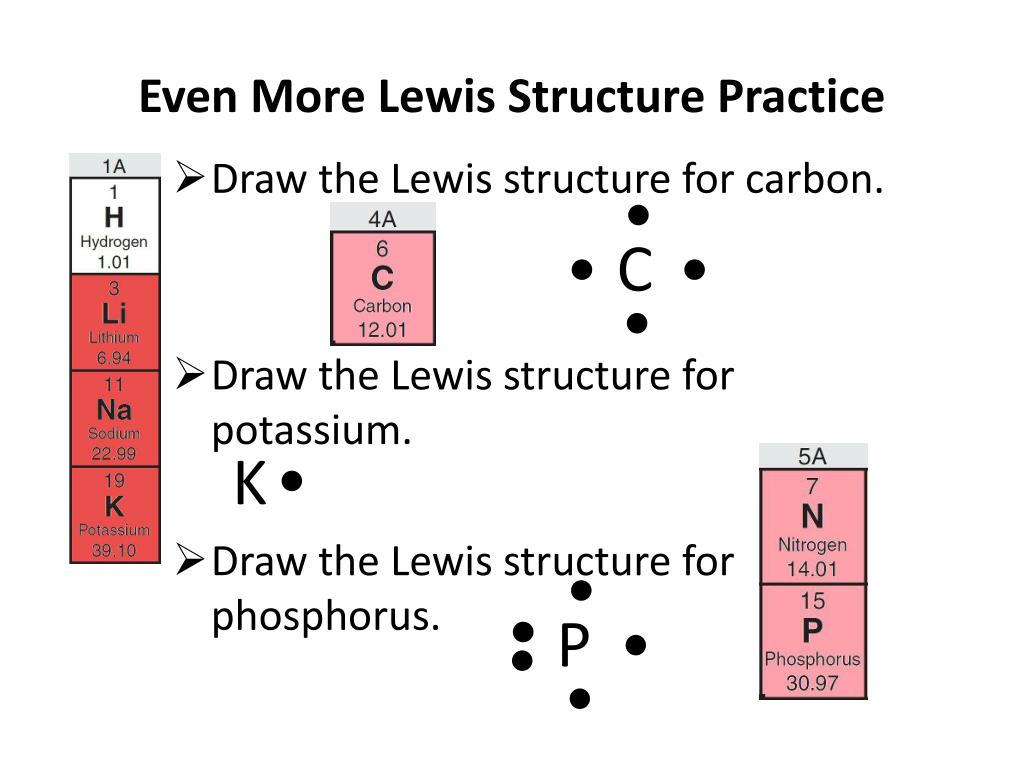
Lewis dot diagrams. Lewis used dots to represent valence electrons. Lewis dot diagrams (see Figure 1) are a quick and easy way to show the valence electron configuration of individual atoms where no bonds have yet been made. The dot diagrams can also be used to represent the molecules that are formed when different species bond with one

Potassium electron dot diagram
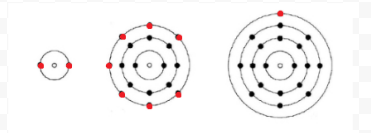
Figure (PageIndex1) The figure over shows the electron shells of that (Helium), Cl (Chlorine), and K (Potassium) and also their Lewis dot structures below. An alert how both the electron shell and the lewis dot structures have actually the same variety of valence electrons. The lewis dot structure ignores the nucleus and all non-valence ... Potassium permanganate, also known as permanganate of potash or Condy's crystals, is a chemical compound with a chemical formula of KMnO4, made of a potassium (K+) ion and a permanganate (MnO4-) ion. 1 day ago · Now, we will proceed to draw a simple sketch or skeletal diagram of cyanide ion. We have placed both the carbon and nitrogen atoms as atomic symbols here. We will henceforth place the electron dot notations. We have put all the 10 valence electrons surrounding the constituent atoms of the CN ionic molecule. Here comes the concept of octet ...
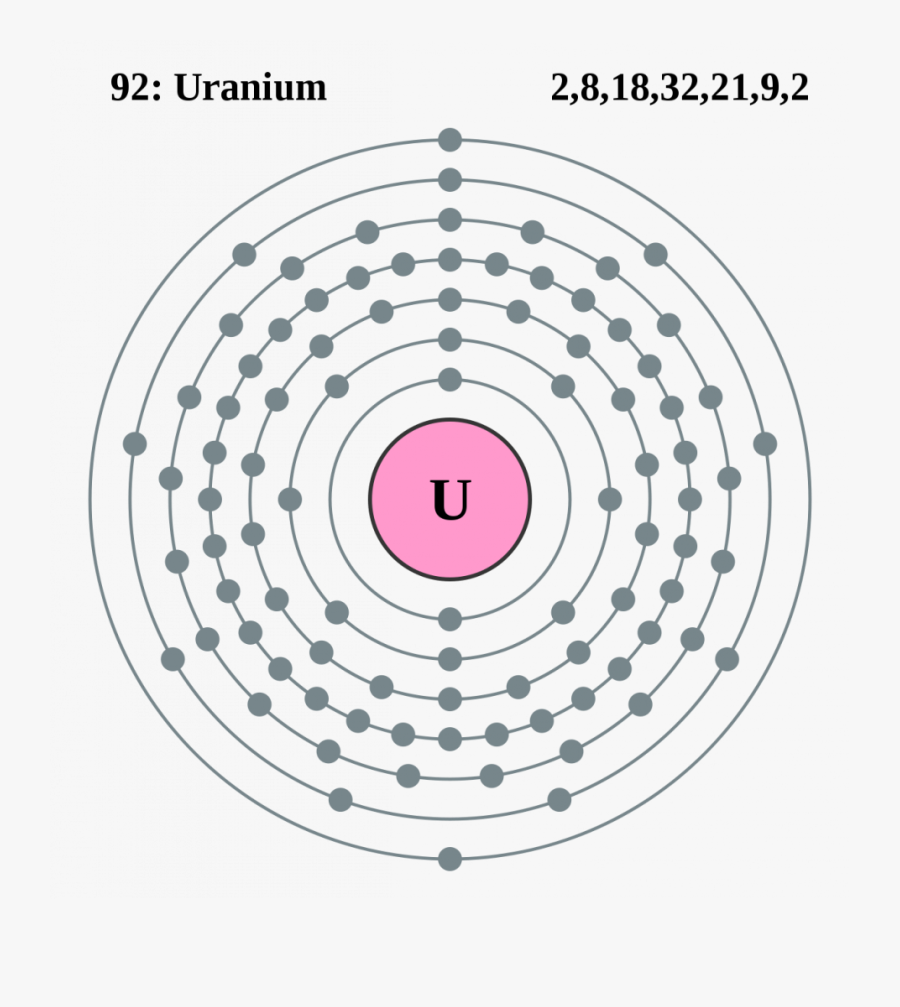
Potassium electron dot diagram. An electron dot diagram is a representation of an atom's valence electrons that employs dots to surround the element's symbol. The number of dots corresponds to the atom's valence electrons. With no more than two dots on each side, these dots are positioned to the right and left, above and below the symbol. Determine the number of valence electrons in the atoms below. and draw dot ... 7. potassium ... Draw Lewis dot diagrams of the following ions and atoms.2 pages For example, potassium has 19 electrons Draw a small circle and write the symbol in the centre. This represents the nucleus Draw a circle around the nucleus. This is the first electron shell Add up to two electrons to the first electron shell. Electrons are usually represented by a dot or cross Draw another circle around the first shell. Here are a number of highest rated Calcium Oxide Dot Diagram pictures upon internet. We identified it from obedient source. Its submitted by handing out in the best field. We believe this nice of Calcium Oxide Dot Diagram graphic could possibly be the most trending topic in imitation of we portion it in google help or facebook.
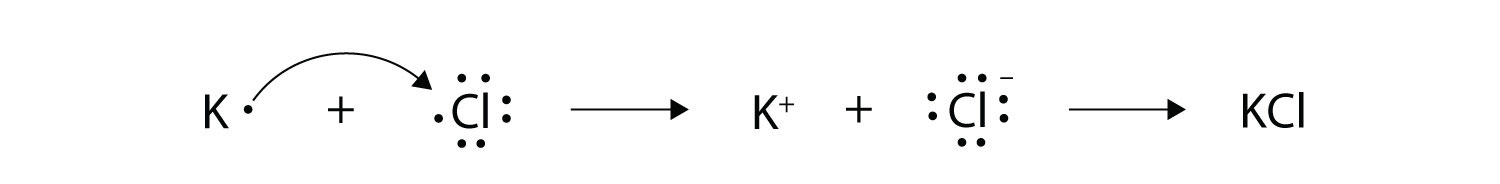
Here, one electron is released by potassium which is accepted by bromine element. In this process, Potassium becomes cation having +1 charge and Bromine become anion having (-1) charge. The ionic equation follows: The electron dot structure is provided in the image below. Electron Configuration and Dot Structure for Calcium … Source: hi-static.z-dn.net. What Is The Ground State Electron Configuration Of Calcium …. This means that in a neutral calcium atom, there are 20 protons in its nucleus. And the electron configuration is the standard notation used to describe the electronic structure of an atom. As you can see, potassium has one valence electron located on the fourth energy level in the 4s-orbital. This means that its electron dot diagram will feature its chemical symbol and one dot, usually placed above the symbol. Is K an anion or cation? Positive and Negative Ions: Cations and Anions Sodium chloride magnesium sulfide calcium fluoride potassium oxide beryllium phosphide strontium bromide barium nitride potassium iodide lithium bromide page 2 of 2 3. The video covers the basic lewis structures for a general chemistry class. Covalent Compound Lewis Structure Worksheets Teaching Chemistry Chemistry Worksheets Chemistry Classroom Department of chemistry university of texas at ...
Since Potassium has 1 electron in its outermost shell , so its electron dot structure involves K with one dot . What is the structure of potassium chloride? The crystal structure of potassium chloride is like that of NaCl. It adopts a face-centered cubic structure. Its lattice constant is roughly 6.3 Å. Crystals cleave easily in three directions. Electrochemiluminescence (ECL) is the process where species generated at electrodes undergo electron-transfer reactions to form excited states that emit light. Application of a voltage to an electrode in the presence of an ECL luminophore such as Ru(bpy) 3 2 + (where bpy = 2,2′-bipyridine) results in light emission and detection of the emitter at very low concentrations (<10 −11 M). Electron dot diagram of Carbon atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Carbon, we got to know, it has 4 valence electrons. So, just represent these 4 valence electrons around the Carbon atom as a dot. For each molecule on the worksheet the lewis dot structure the number of valence electrons the electron arrangement e a and the molecular geometry m g are given respectively. B Worksheet Answers 4 2 Ib Chemistry Worksheet 4 2 Lewis Dot Diagrams 1 Bsf 2 Hbr 3 C2h5oh Ethanol 4 N2f4 5 Sf6 Yet More Lewis Structures Answers For Course Hero.
Bohr Diagram. Lewis Dot Structure. Ne. electron config # protons. #electrons. charge. Bohr Diagram. Lewis Dot . Making Ions - Ionic Bonds are made of Ions. A strong understanding of Ions is needed. Notes: Remember that Metals tend to lose their electrons, falling back to their inner octet, becoming smaller, forming positive "cations".
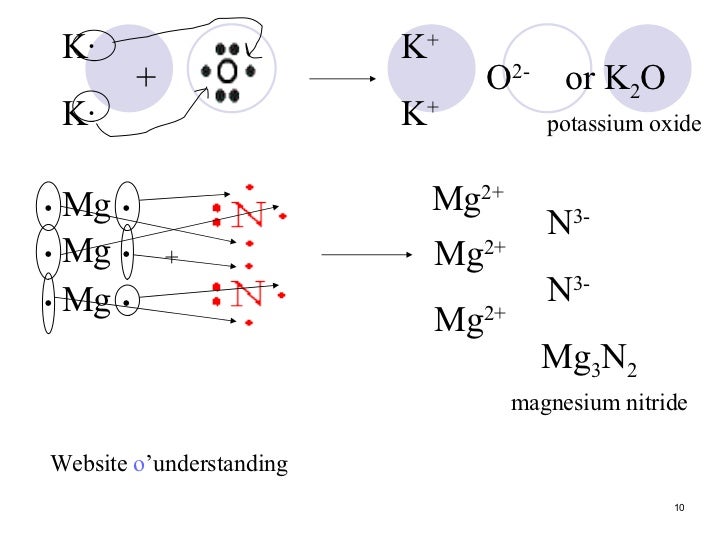
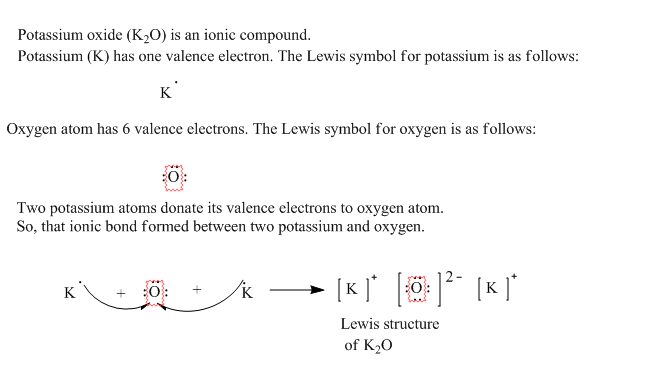
Potassium has a 1+ charge, and oxygen has a 2− charge. a lewis dot diagram should contain two potassium atoms. Potassium has a 1+ charge, and oxygen has a 2− charge. a lewis dot diagram should contain two potassium atoms and one oxygen atom to show how these atoms form an ionic bond. true or false.
Sodium chloride magnesium sulfide calcium fluoride potassium oxide beryllium phosphide strontium bromide barium nitride potassium iodide lithium bromide page 2 of 2 3. Show how lewis dot diagrams also represent ionic bonding. Chemistry worksheet lewis dot structures name. Compare and contrast covalent and ionic compounds with regard to.
Potassium has one electron in its outer shell, and Sulfur has two. When forming an ionic compound with one atom of sulfur, two potassium atoms are used. This is an ionic bond and is the basis of the ionic compound formed, as the differently charged ions are held together by their opposite charges.
Jul 25, 2018 — Potassium : As it belongs to s -block , the electronic configuration of potassium is 1s² 2s² 2p⁶ 3s² 3p⁶4s¹ .Since Potassium has 1 electron in ...2 answers · Top answer: Answer : The correct answer for ions present in KCl is K⁺ and Cl⁻ which forms KCl by ionic ...
Thus the three atoms shown in Figure 1 from Electrons and Valence can be represented by the following Lewis diagrams: Figure 5.3. 1 The figure above shows the electron shells of He (Helium), Cl (Chlorine), and K (Potassium) as well as their Lewis dot structures below. Notice how both the electron shell and the lewis dot structures have the same ...
Electron Dot Diagrams. As valence electrons are significant to an atom's reactivity, it is important to represent them by simple diagrams. Lewis structures, here, comes into the picture where the valence electrons present in an atom are represented as dots. These structures are also known as electron dot diagrams.
Electron dot structure of KCLO3 - Chemistry - - 9869219 ... Kclo3 buy potassium chlorate from China For the following reaction KClO3 ---->KCl+3/2 O2 assign ...
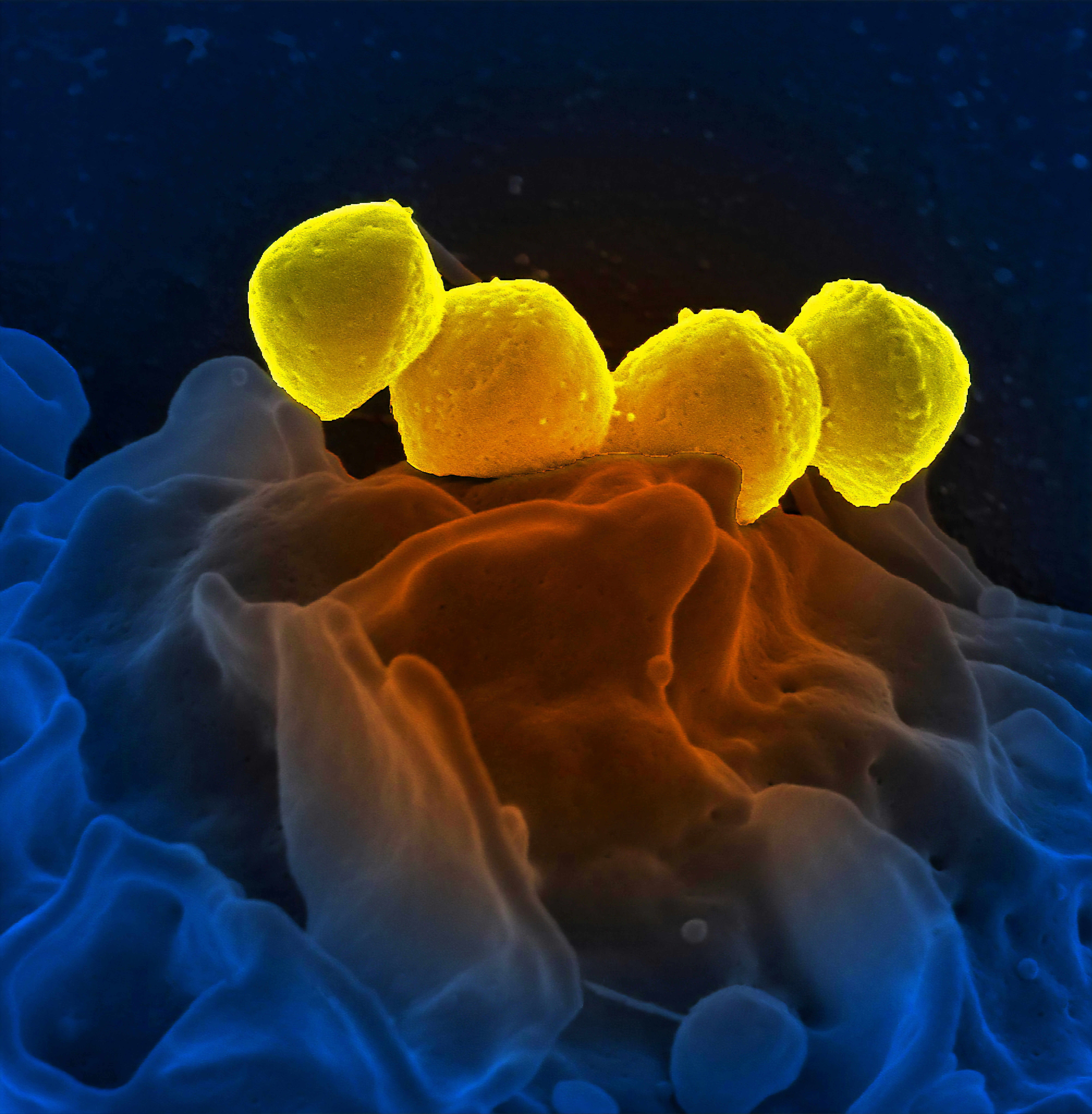
Produced by the National Institute of Allergy and Infectious Diseases (NIAID), this digitally colorized scanning electron microscopic (SEM) image depicts four, yellow colored, Group A Streptococcus (GAS), Streptococcus pyogenes bacteria, which were atop the surface of a human white blood cell (WBC), known as a neutrophil.
(iii)Electron dot Orbit structure diagram of CaO: The Calcium ion is an Alkaline earth metal and wants to give up the 2 s orbital elections and become a +2 cation. Oxygen has six valence electrons and is looking to gain two electrons to complete the octet (8) electron count in the valence shell making it a -2 anion.
What is the Lewis structure of Cl2? For example, when two chlorine atoms, each with 7 valence electrons, come together to form a diatomic chlorine molecule, the Lewis structure shows that there will be a sharing of two electrons between the two chlorine atoms which allows both chlorine to be surrounded by 8 electrons….Lewis Dot Structures.
The schematic diagram in Fig. 6d shows the separation and reaction process of the photoexcited electron–hole in the Nv-CNN. The PL spectra, in combination with experimental characterization, lead to the conclusion that the enhanced photocatalytic activity of Nv–CNN–3 is mainly owing to the proper content of N vacancies.
Lewis dot structures and molecule geometries worksheet answer key 1 lewis dot structures and molecule geometries worksheet answer key how to draw a lewis dot structure 1. Draw a lewis dot diagram and answer the questions for the following elements student exploration ionic bonds answer key quizlet. Explain its role in bonding between atoms.
Potassium oxide (K 2 O) is also an ionic compound. It is made of two potassium atoms and one oxygen atom. Can you draw the Electron Dot structure of potassium and oxygen atoms? Can you show the formation of potassium oxide? Let us now try to find out the properties of ionic compounds by performing the following activities.
1 day ago · Now, we will proceed to draw a simple sketch or skeletal diagram of cyanide ion. We have placed both the carbon and nitrogen atoms as atomic symbols here. We will henceforth place the electron dot notations. We have put all the 10 valence electrons surrounding the constituent atoms of the CN ionic molecule. Here comes the concept of octet ...
Potassium permanganate, also known as permanganate of potash or Condy's crystals, is a chemical compound with a chemical formula of KMnO4, made of a potassium (K+) ion and a permanganate (MnO4-) ion.
Figure (PageIndex1) The figure over shows the electron shells of that (Helium), Cl (Chlorine), and K (Potassium) and also their Lewis dot structures below. An alert how both the electron shell and the lewis dot structures have actually the same variety of valence electrons. The lewis dot structure ignores the nucleus and all non-valence ...
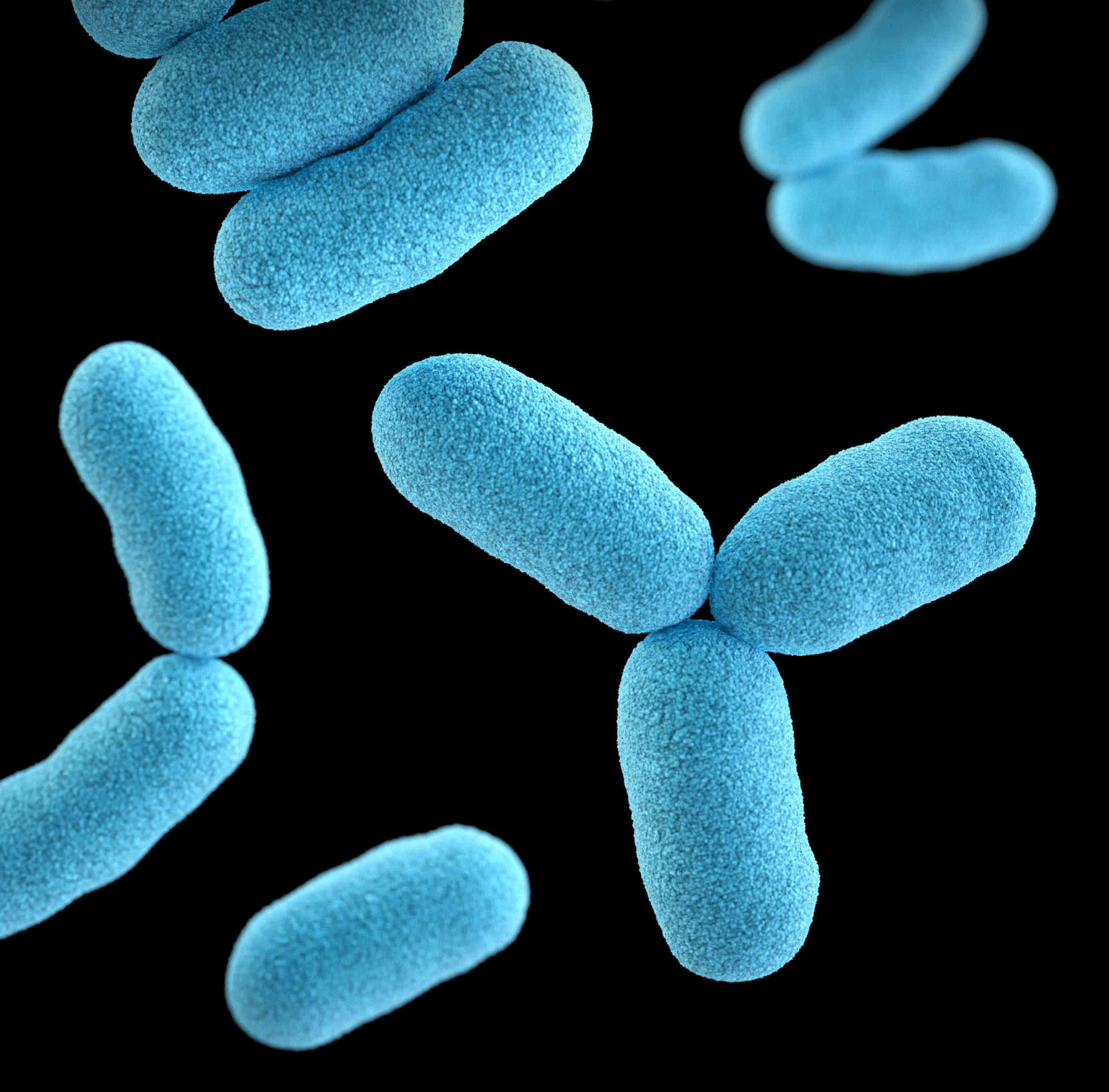
This illustration depicted a three-dimensional (3D), computer-generated image, of a group of Gram-positive, Corynebacterium diphtheriae, bacteria. The artistic recreation was based upon scanning electron microscopic (SEM) imagery.
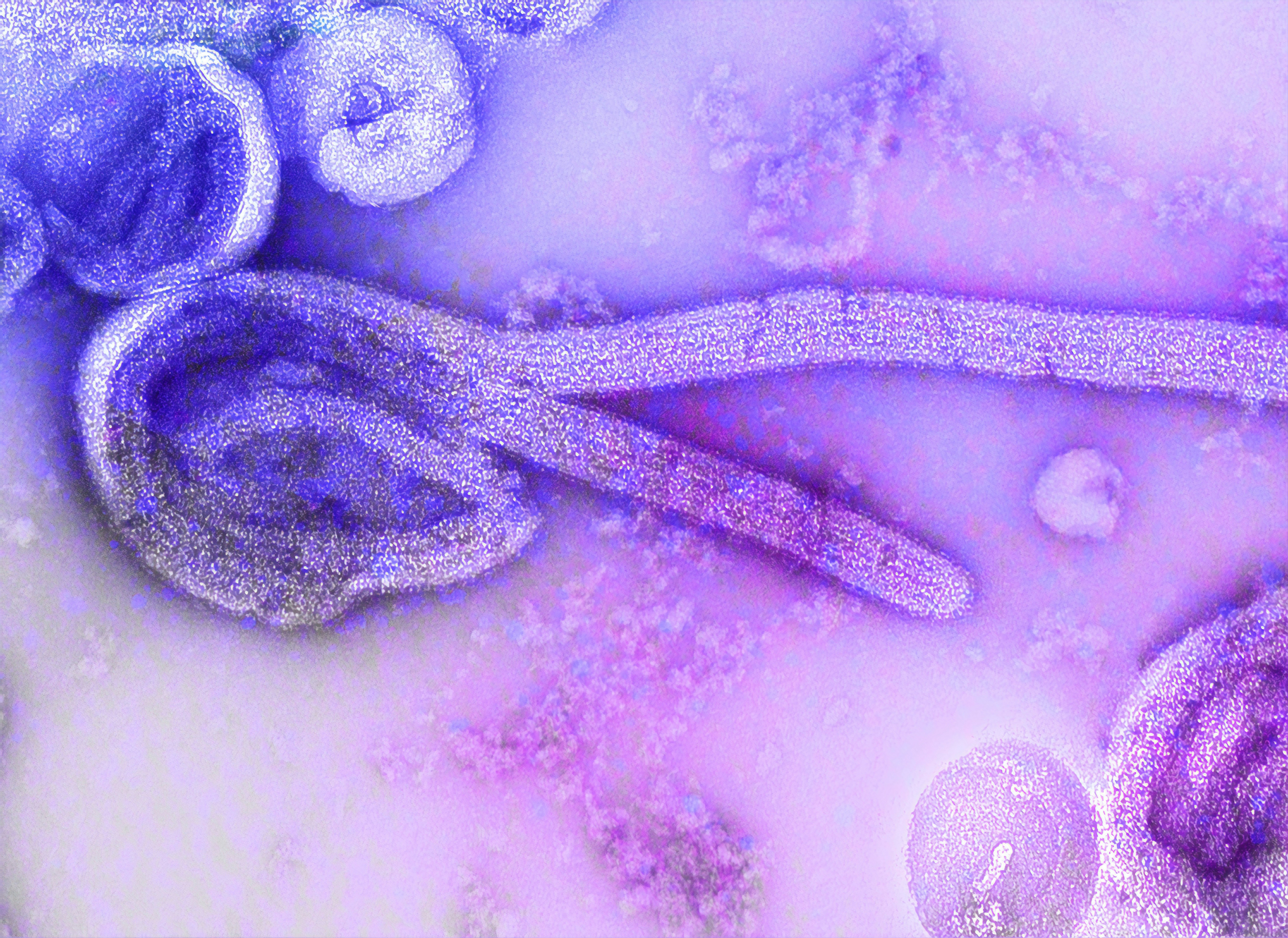
This is an electron microscopic image of the 1976 isolate of Ebola virus. The internal structures of the filamentous particle are visible, including the nucleocapsid and other structural viral proteins, and the outer viral envelope is covered with surface projections. The characteristic “6-shape†of the virus is evident. See PHIL 23185, for a black and white version of this image.
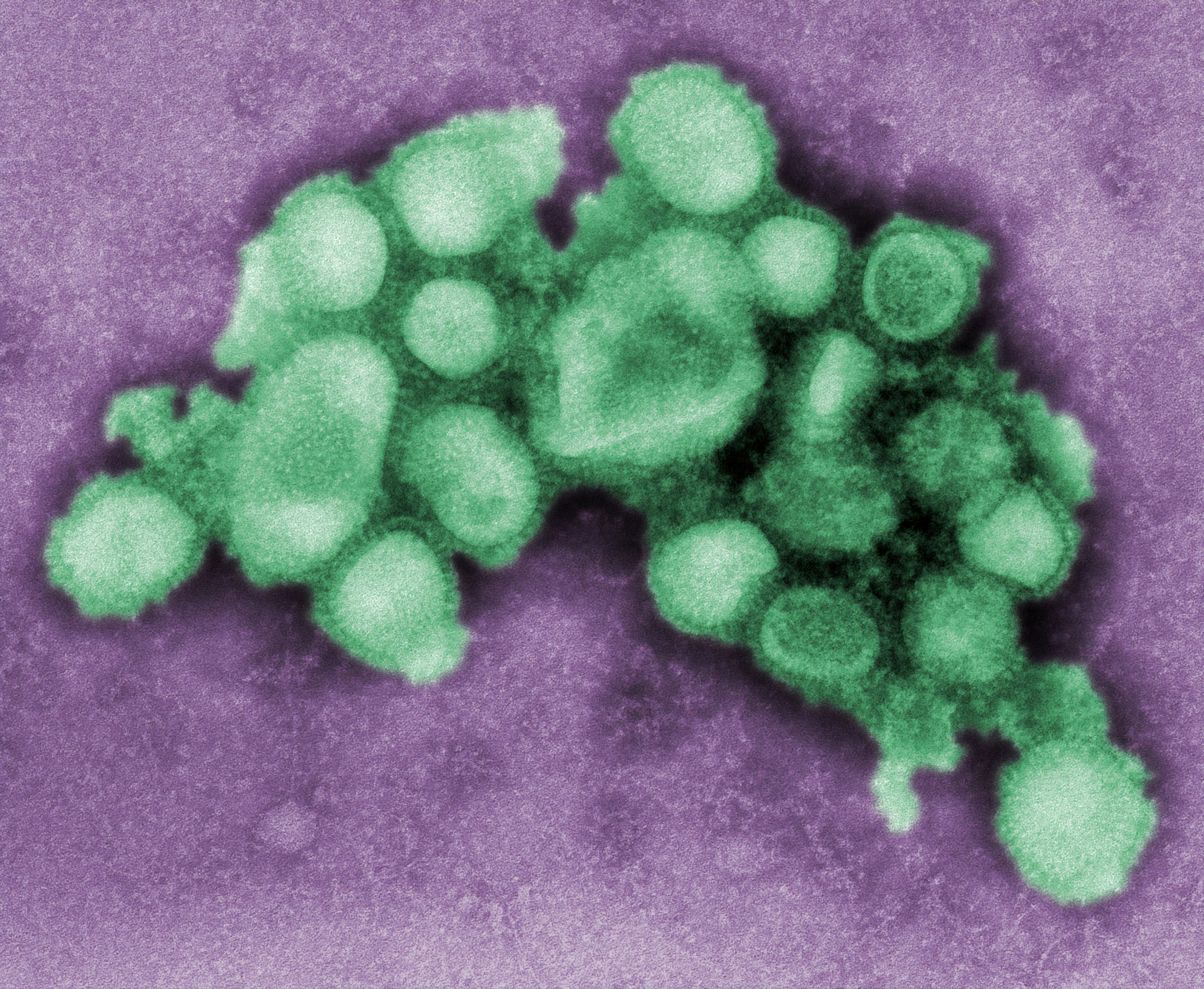
This digitally-colorized, negative-stained transmission electron microscopic (TEM) image depicted some of the ultrastructural morphology of the A/CA/4/09 Swine Flu virus.










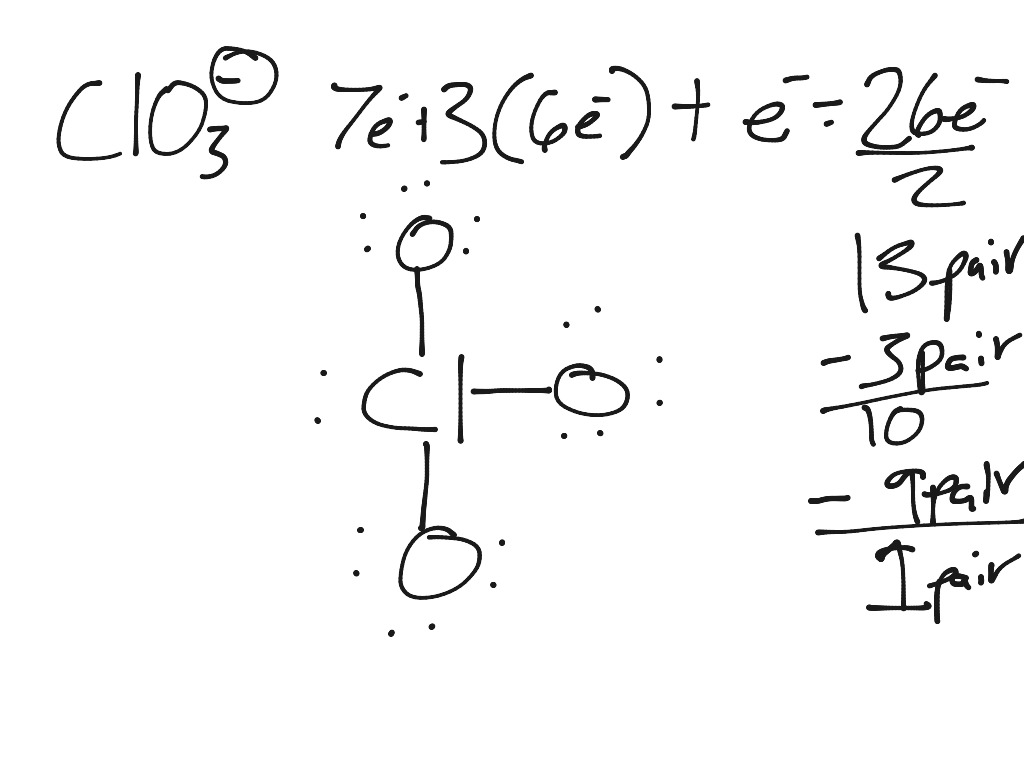





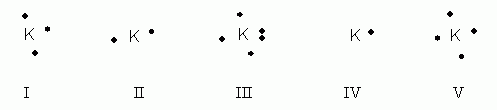
0 Response to "41 potassium electron dot diagram"
Post a Comment