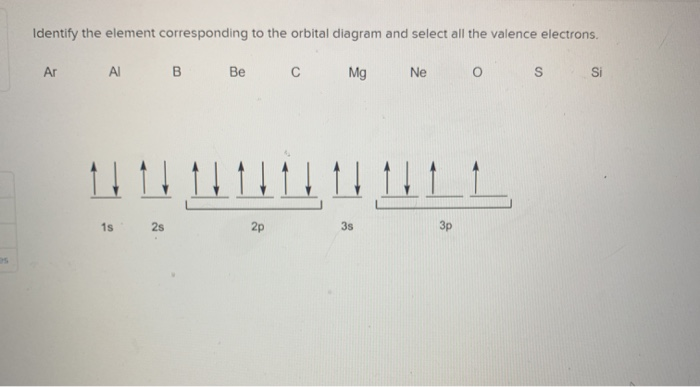
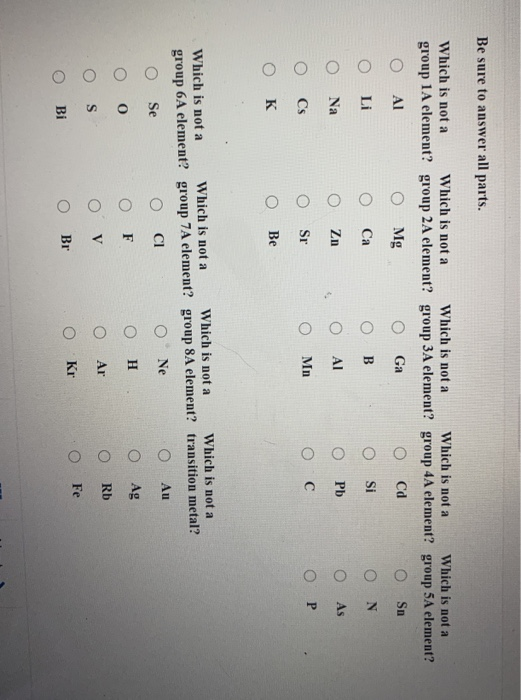
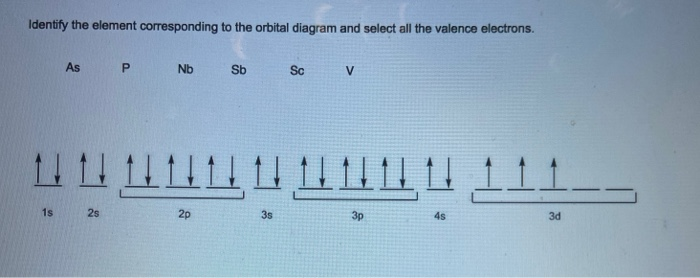
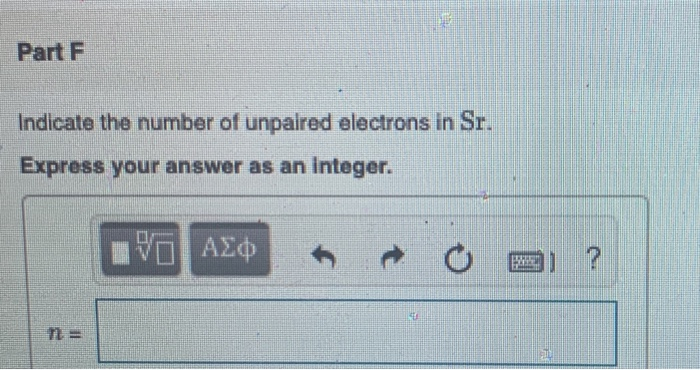
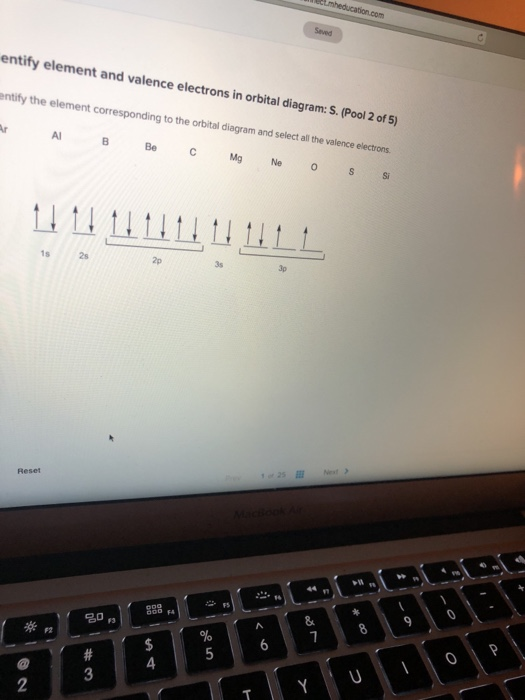
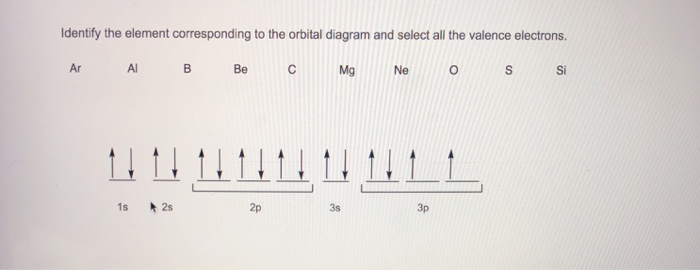
40 identify the element corresponding to the orbital diagram and select all the valence electrons.
Academia.edu is a platform for academics to share research papers. electron configuration and orbital filling diagram. valence electrons. electron dot diagram . common ions, including electron configurations for ions. physical properties, including boiling point, melting point, electrical conductivity, density, atomic radius, shielding effect and ionization potentials, and electronegativity. chemical properties, including reactivity in oxygen, water, and ...
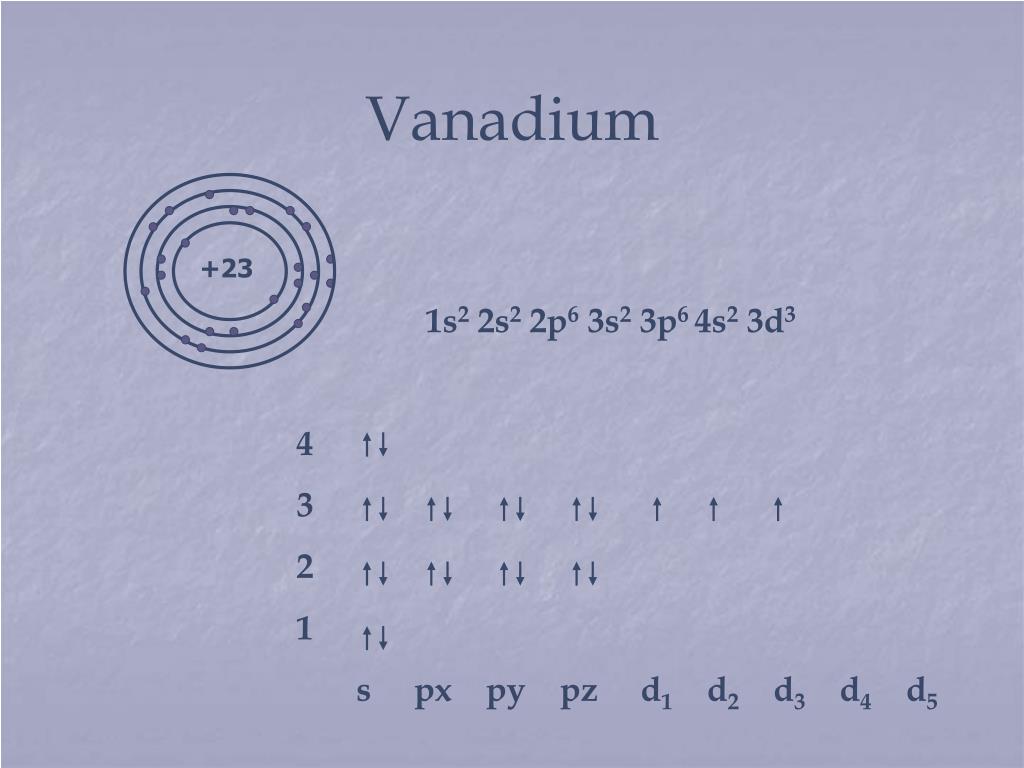
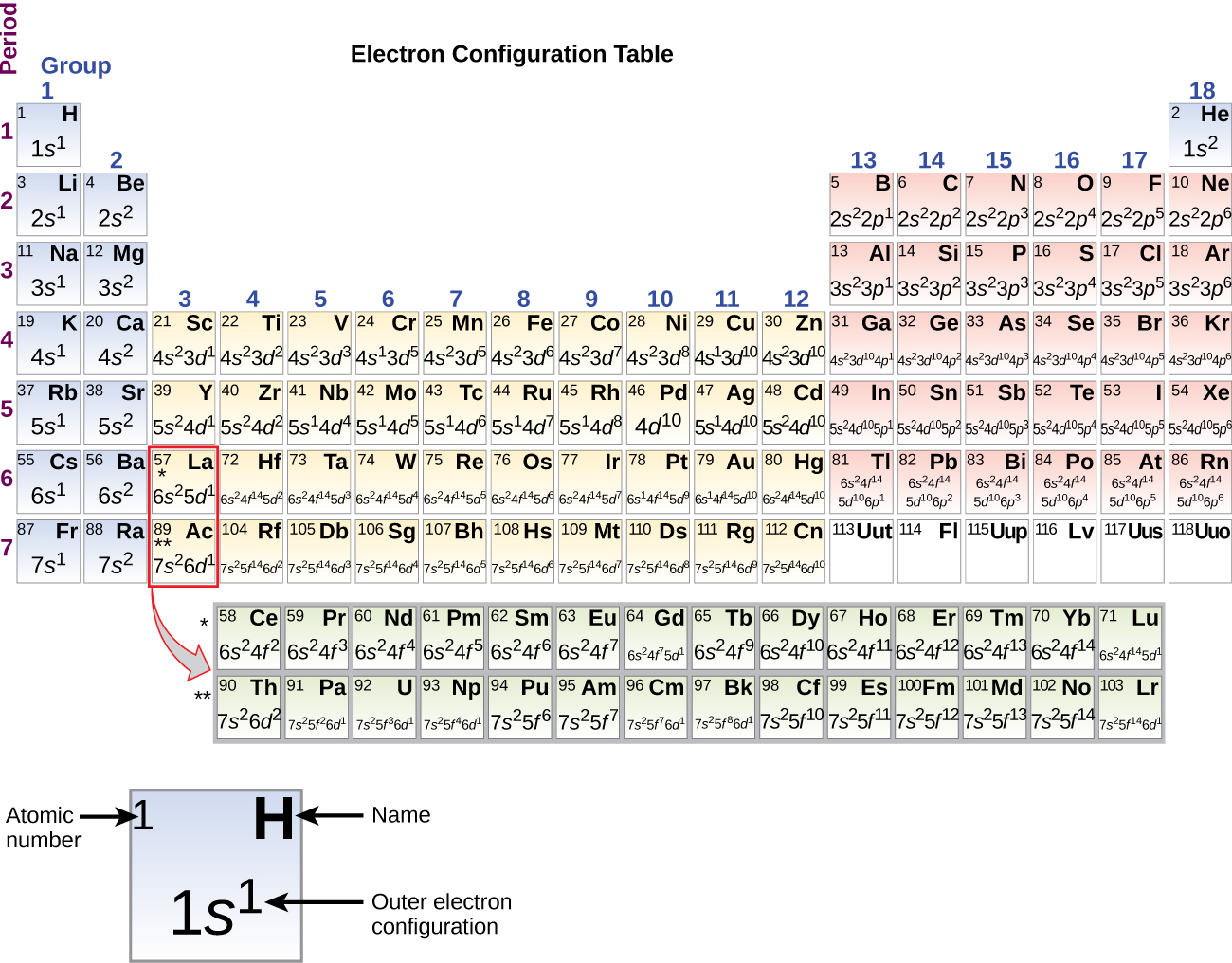
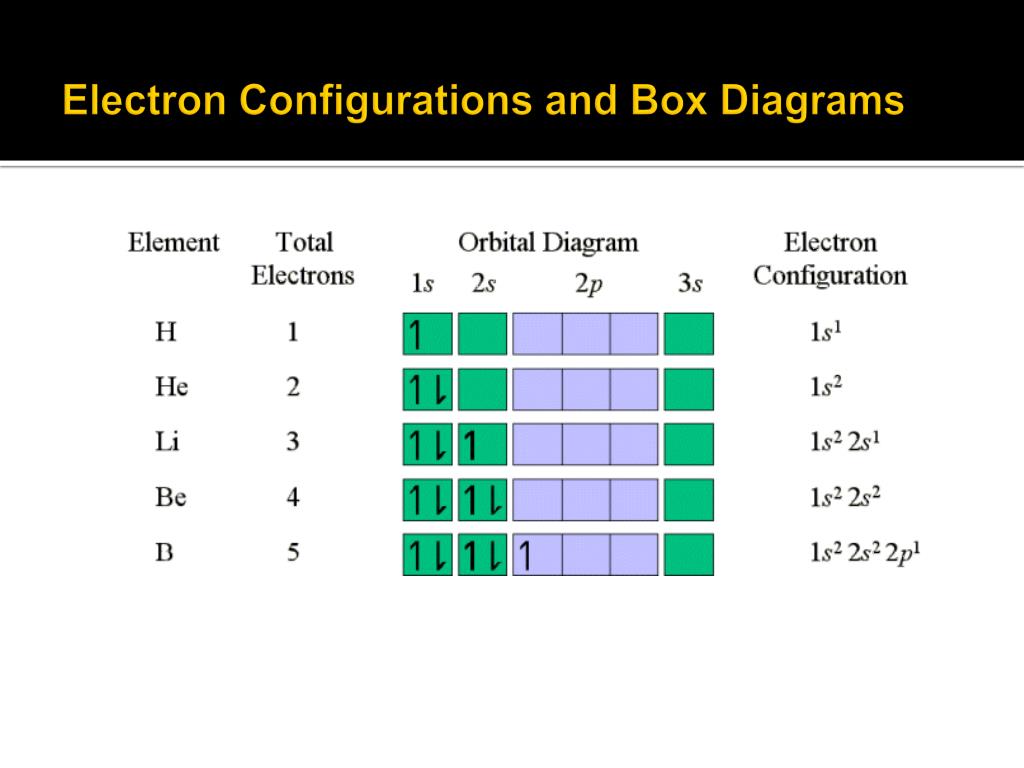
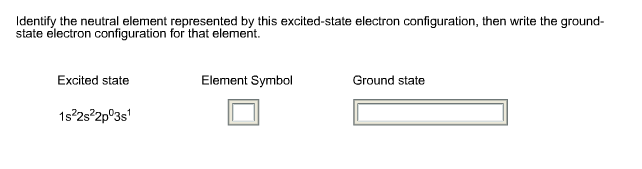
30.06.2015 · Electronic configuration The distribution of electrons into orbitals of an atom is called its electronic configuration. Electronic configuration can be represented in two ways:- (a)Normal notation and (b) orbital diagram As given in the textbook. The electron in the completely filled electronic shell with the highest principal quantum number are called valence …
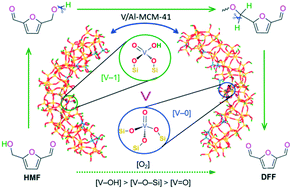
Identify the element corresponding to the orbital diagram and select all the valence electrons.
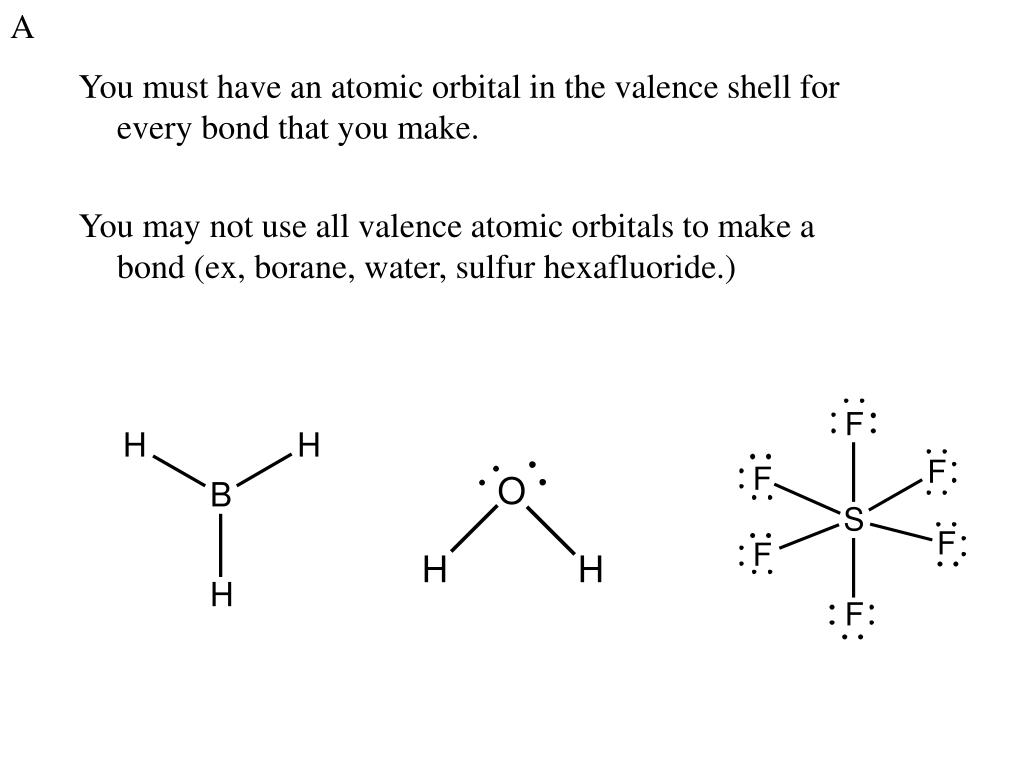
In O2 and F2, the 𝜋2𝑝 orbitals are higher in energy than the 𝜎2𝑝 orbital as shown in diagram B. The number of valence electrons per atom of an element is related to the group number in the periodic table. Boron, in group 3A, has 3 valence electrons per atom. Diatomic boron has 2(3)=6 valence electrons. When you assign us your assignment, we select the most qualified writer in that field to handle your assignment. We do not offer pre-written essays. All our essays and assignments are written from scratch and are not connected to any essay database. Every essay is written independent from other previously written essays even though the essay question might be similar. We also … When you assign us your assignment, we select the most qualified writer in that field to handle your assignment. We do not offer pre-written essays. All our essays and assignments are written from scratch and are not connected to any essay database. Every essay is written independent from other previously written essays even though the essay question might be similar. We also …
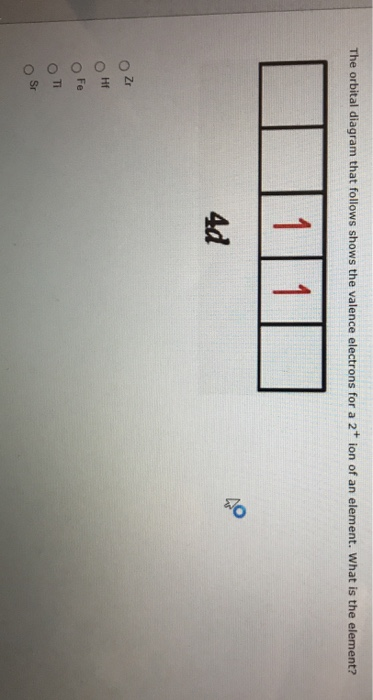
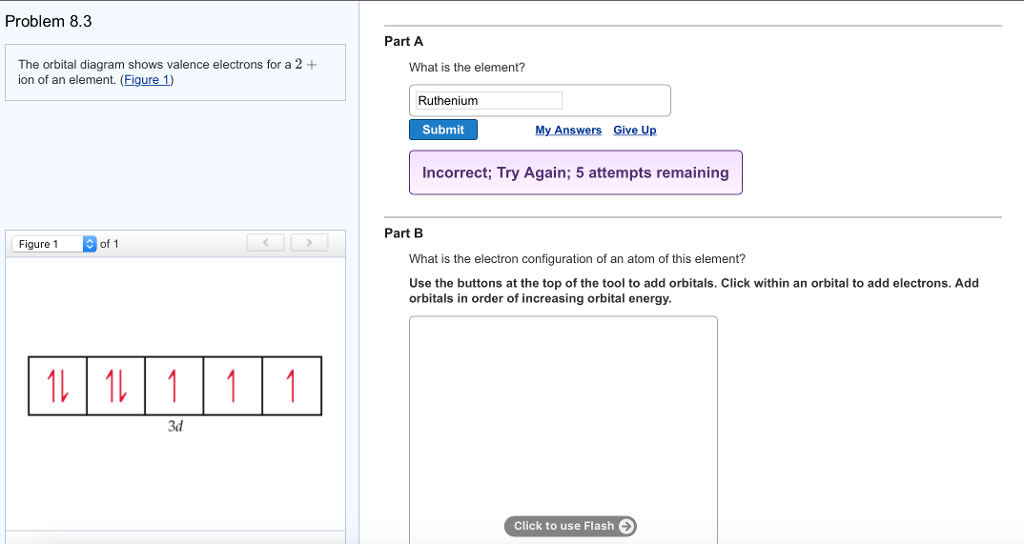
Identify the element corresponding to the orbital diagram and select all the valence electrons.. From this point through element 71, added electrons enter ... have now become so large that the oxidation state corresponding to the formal loss of all valence electrons is encountered only rarely (group 8) or not at all (groups 9 and 10). As a result, the chemistry of all three groups is dominated by intermediate oxidation states, especially +2 and +3 for the first-row metals (Fe, … 8.4 The orbital diagram that follows shows the valence electrons for a 2+ ion of an element. (a) What is the element? (b) What is the electron configuration of an atom of this element? [Section 8.2] 8.5 In the Lewis structure shown here, A, D, E, Q, X, and Z represent elements in the first two rows of the periodic table (H− Ne). Identify all six elements so that the formal charges of all ... Select all that apply. l, ms. Which of the following signifies the spatial orientation, i.e. direction, of an atomic orbital? magnetic quantum number, ml. Which set of quantum numbers is invalid? n=1, l=1, ml=0, ms=12. Electrons that inhabit different orbitals must have a different value for the: none of above They could have the same principle quantum number if they are in different orbitals ... The tutorials are listed in alphabetical order, but if you are new to electronics, I would recommend to start with DC Theory. Wayne Storr has created a very good set of tutorials, ranging from DC- and AC-Theory over the basic devices Resistor,
When you assign us your assignment, we select the most qualified writer in that field to handle your assignment. We do not offer pre-written essays. All our essays and assignments are written from scratch and are not connected to any essay database. Every essay is written independent from other previously written essays even though the essay question might be similar. We also … When you assign us your assignment, we select the most qualified writer in that field to handle your assignment. We do not offer pre-written essays. All our essays and assignments are written from scratch and are not connected to any essay database. Every essay is written independent from other previously written essays even though the essay question might be similar. We also … In O2 and F2, the 𝜋2𝑝 orbitals are higher in energy than the 𝜎2𝑝 orbital as shown in diagram B. The number of valence electrons per atom of an element is related to the group number in the periodic table. Boron, in group 3A, has 3 valence electrons per atom. Diatomic boron has 2(3)=6 valence electrons.
















0 Response to "40 identify the element corresponding to the orbital diagram and select all the valence electrons."
Post a Comment