39 v5+ orbital diagram
take a look here at utilizing the periodic table too. Do some orbital configurations or diagrams for some metal cat ions. So first example is going to be for c… Determine if the ion is diamagnetic or paramagnetic.a. V5+b. Cr3+c. Ni2+d. Fe3+. FREE Expert Solution Show answer.
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of .. Rb+, Se2−. The first orbital (an s orbital) can contain only two electrons.. Rubidium.

V5+ orbital diagram
Acer Aspire V5 431. The first page 431 motherboard schematic Diagram of the initial specifications that you can file schematic number, Model Name motherboard, chipset and… to view. The second page of the laptop schematic and connections General Block Diagram related to the page. Orbital Diagram for Vanadium - YouTube. Example of following the Aufbau principle, Pauli principle, and Hund's rule to construct an orbital diagram for a vanadium (Z=23) atom. Orbital diagrams are like the configuration notation just introduced, except with the spins of electrons indicated. Use the Pauli exclusion principle and Hund's rule to work out how to fill shells. The exclusion principle states that no two electrons can share the same four quantum numbers, which basically results in pairs of states containing electrons with opposite spins.
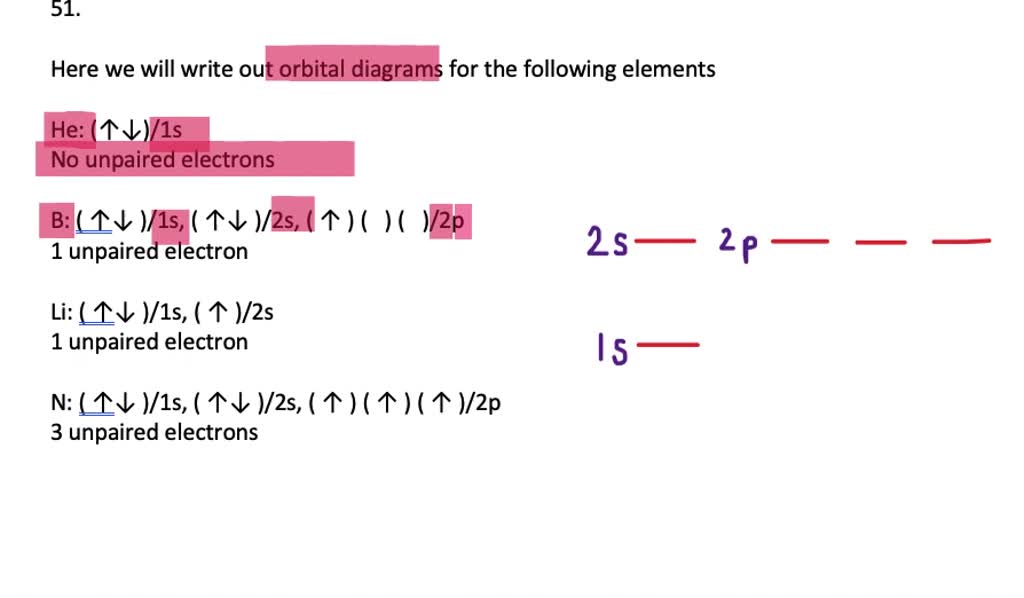
V5+ orbital diagram. Q: Consider the following two reductions:Zn+2 + 2e‾→Zn E°red = -0.76 VCr+3 + 3e‾ → Cr ... A: Since the cell reaction should be such that the net cell voltage is positiveAnd since on subtractin... question_answer. Q: An aqueous solution is 4.00% ammonium chloride, NHĄ Cl, by mass. Consider the electron configurations and orbital diagrams for elements with atomic numbers 3–10. Notice that, as a result of Hund's rule, the p orbitals ... Electrons don't pair up in orbitals of equal energy until they have to, and all electrons in singly occupied orbitals have the same spin. orbital diagram for nitrogen orbital diagram for fluorine Q. Write orbital diagrams for each of these ions.V5+ Q. Write orbital diagram to represent the electron configurations-without hybridization-for F in SF2. Q. Choose the correct orbital diagram for vanadium. See all problems in The Electron Configuration: Ions Frequently Asked Questions What scientific ...
V5+ orbital diagram" Keyword Found Websites Listing . Keyword-suggest-tool.com DA: 28 PA: 30 MOZ Rank: 70. An orbital diagram naturally leads to the writing of an electron configuration; V5 + Cr3+ Ni2+ Fe3+ Explore each Elements orbitals and electron configuration; It is a powerful fluorinating as well as an oxidizing agent Now I asked to look, were asked also to look at making an orbit diagram and determining whether vanadium as a five plus cat iron is dia or para magnetic in ... Jan 07, 2022 · V5+ orbital diagram. V5+ Orbital Diagram. Since the 4s orbital is higher in energy, its electrons will be removed first. Not that it matters here, though, because exactly 5 electrons are. can be accommodated in the metal d orbitals. • d0 ions – Ti4+, Zr4+, V5+, Ta5+, Cr6+, Mo6+, etc. • d1 ions . σ-ML4 Tetrahedral MO Diagram e. Orbital Diagram For Vanadium (V) | Vanadium Electron Configuration. February 18, 2021 by Sneha Leave a Comment. Vanadium Electron Configuration: When it comes to electronic configuration, it is one of the major topics in chemistry as we have mentioned before in our article.
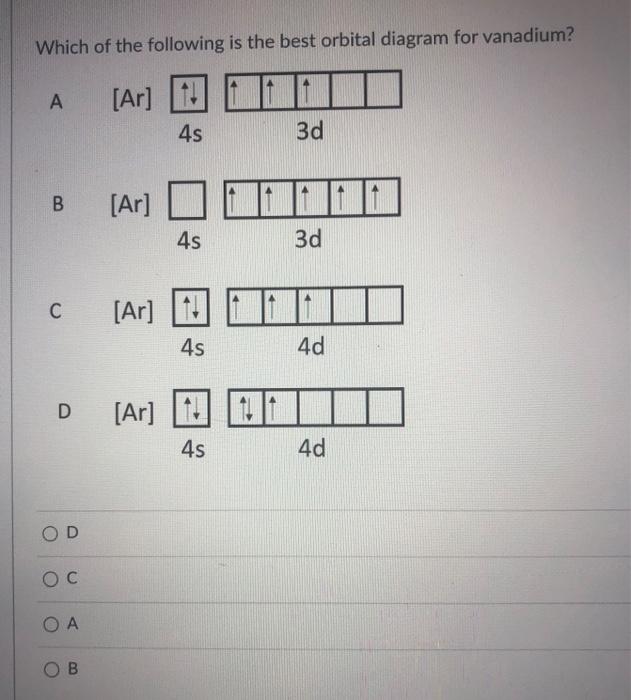
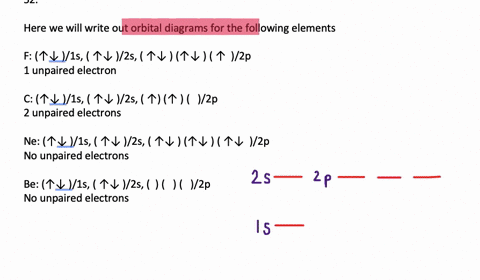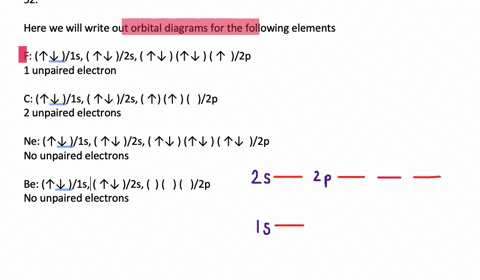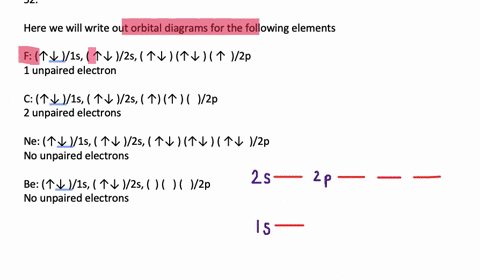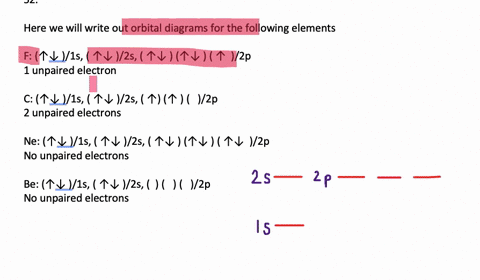
Orbital Diagram. Orbital diagrams are ways to assign electrons in an atom or ion. Each atomic orbital is represented by a line or a box and electrons in the orbitals are represented by half arrows. Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. V5+ b. Cr3+ c. Ni2+ d. Fe3+ Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V5+,Cr3+,Ni2+,Fe3+ Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V 86%(14). Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic. V5+ orbital diagram keyword after analyzing the system ...
To write the configuration for the Vanadium and the Vanadium ion, first we need to write the electron configuration for just Vanadium (V). We first need to ...
Dec 19, 2018 · on V5+ Orbital Diagram. Since the 4s orbital is higher in energy, its electrons will be removed first. Not that it matters here, though, because exactly 5 electrons are. can be accommodated in the metal d orbitals. • d0 ions – Ti4+, Zr4+, V5+, Ta5+, Cr6+, Mo6+, etc. • d1 ions . σ-ML4 Tetrahedral MO Diagram e.
An orbital diagram naturally leads to the writing of an electron configuration. V5+ Cr3+ Ni2+ Fe3+ Explore each Elements orbitals and electron configuration. Xenon (Xe). It is a powerful fluorinating as well as an oxidizing agent. answer choices . For each electron shell atom diagram, the element symbol is listed in the nucleus.
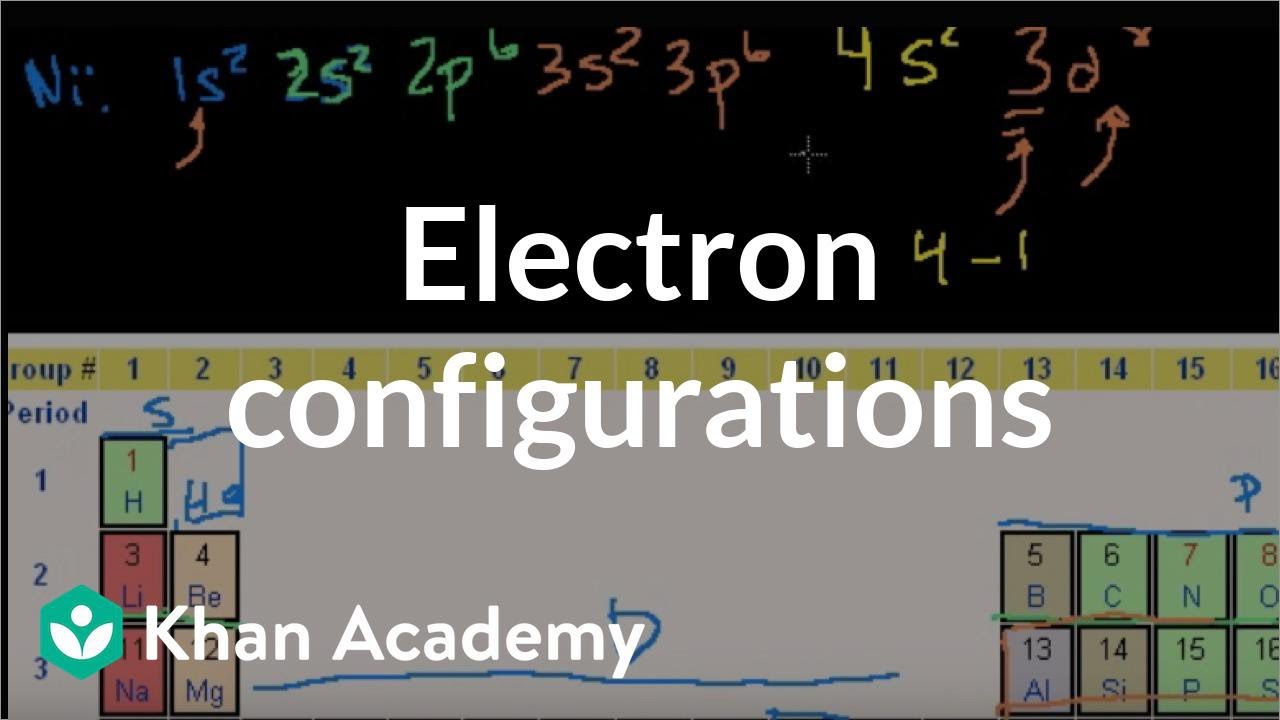
Ni Orbital Diagram Electron configuration elements solved: Write complete electron configurations for (a) State Density (DOS) and (b) Crystal Orbital. Valence electrons remain in one orbital orbit. In other words, once we get to the principle of quantum number 3, the highest subshells of lower quantum eclipse numbers in the energy lowest podshelle of higher quantum numbers: 3 d is higher energy than 4 s.
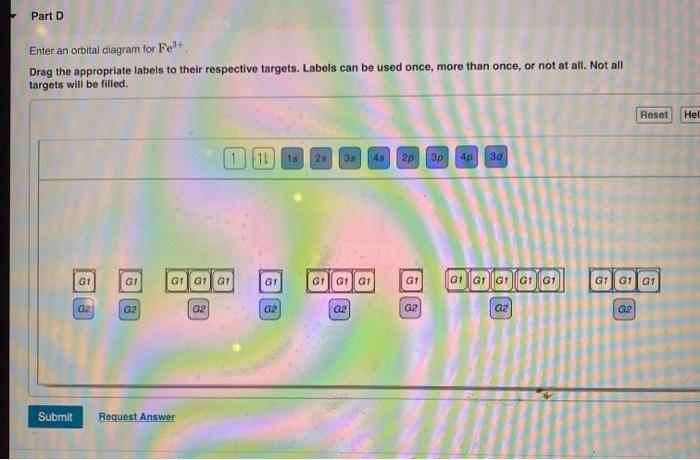
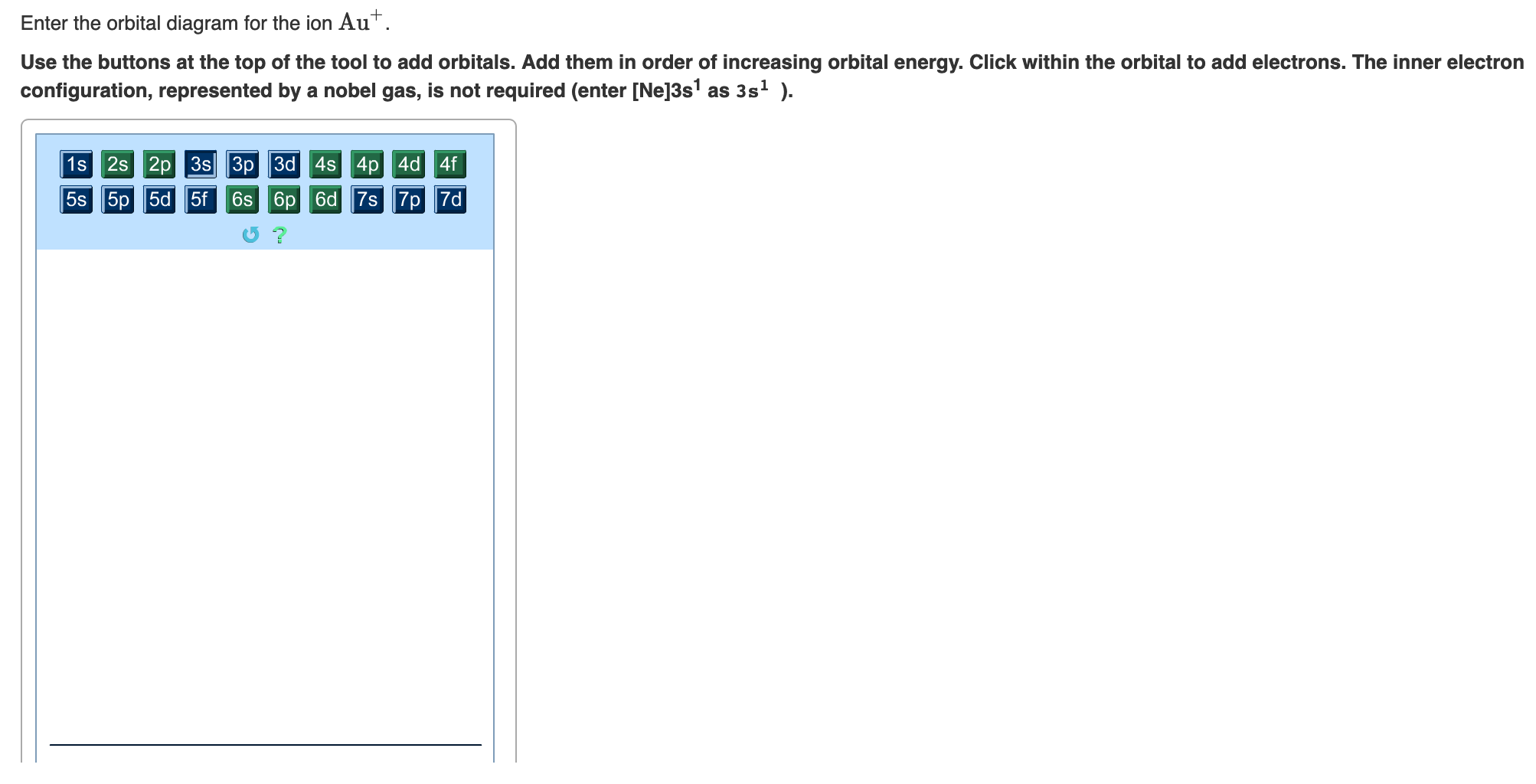
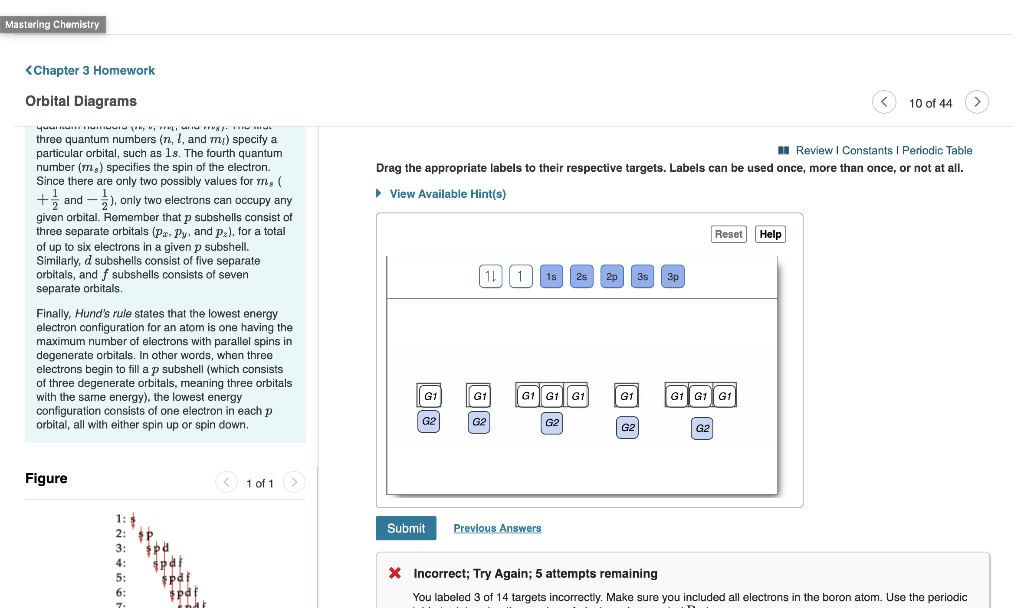
Part A Enter an orbital diagram for V5+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all.
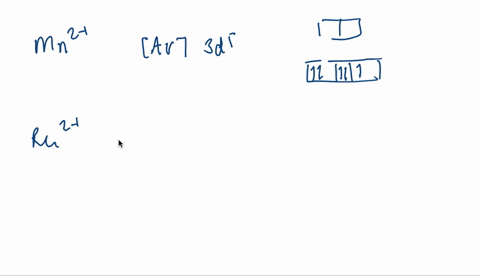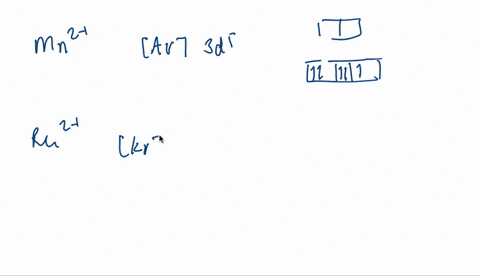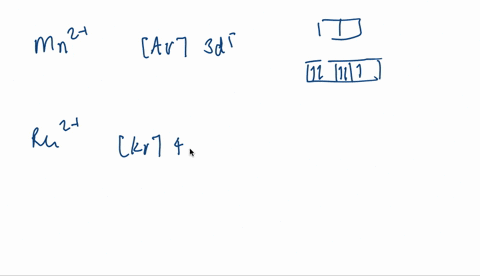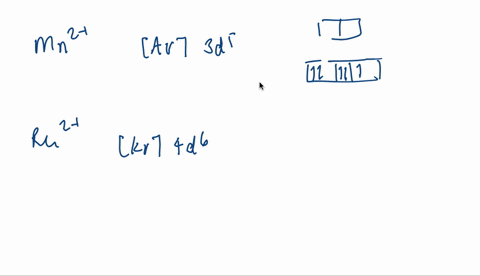
V electron configuration: [Ar] 4 s 2 3 d 3 4s^23d^3 4 s 2 3 d 3. Removing 5 electrons to attain +5 charge, we are left with [Ar]. V. 5 + ^ {5+} 5 +. electron configuration: [Ar], the orbital diagram of [Ar] is. Since there are no unpaired electrons, V. 5 + ^ {5+} 5 +. is diamagnetic.
Orbital diagrams are like the configuration notation just introduced, except with the spins of electrons indicated. Use the Pauli exclusion principle and Hund's rule to work out how to fill shells. The exclusion principle states that no two electrons can share the same four quantum numbers, which basically results in pairs of states containing electrons with opposite spins.
Orbital Diagram for Vanadium - YouTube. Example of following the Aufbau principle, Pauli principle, and Hund's rule to construct an orbital diagram for a vanadium (Z=23) atom.
Acer Aspire V5 431. The first page 431 motherboard schematic Diagram of the initial specifications that you can file schematic number, Model Name motherboard, chipset and… to view. The second page of the laptop schematic and connections General Block Diagram related to the page.




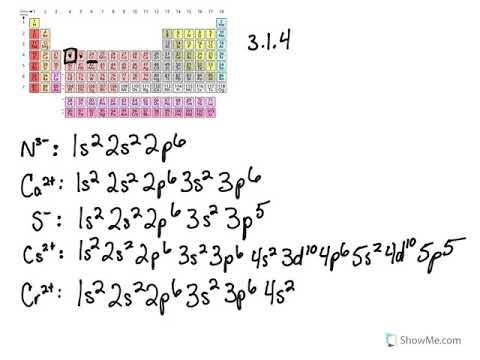



















0 Response to "39 v5+ orbital diagram"
Post a Comment