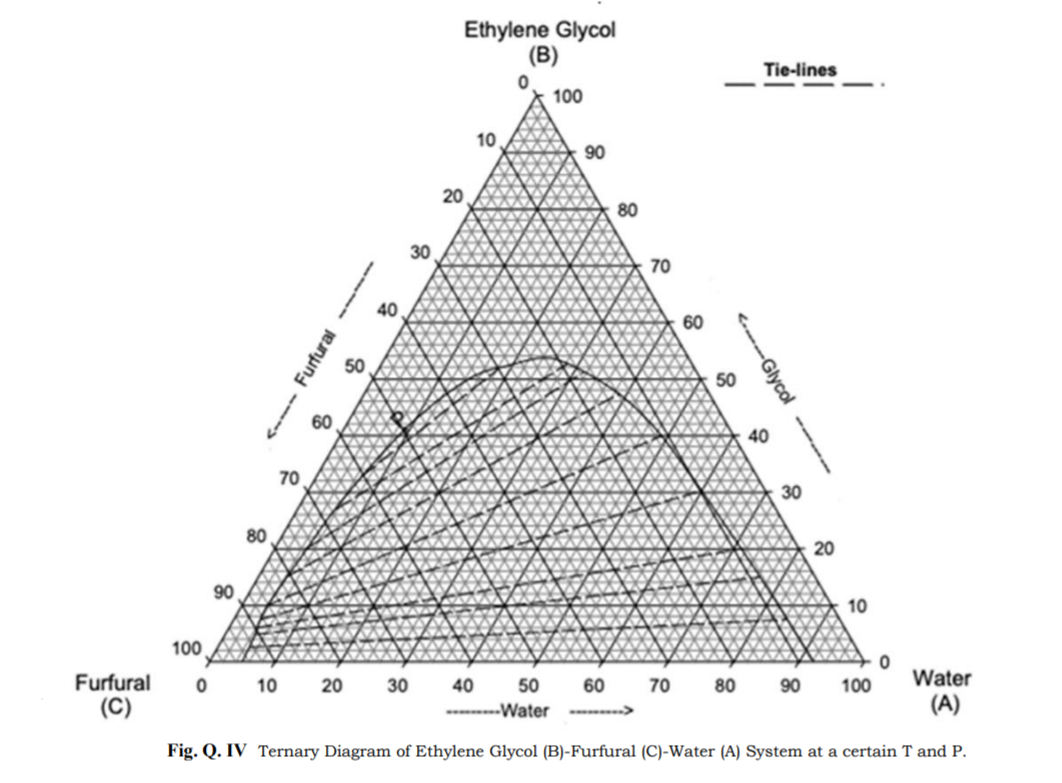
39 tie lines phase diagram
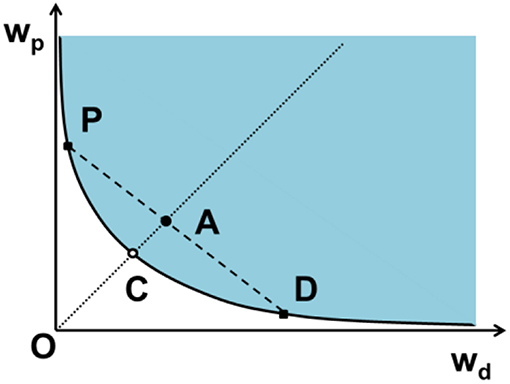
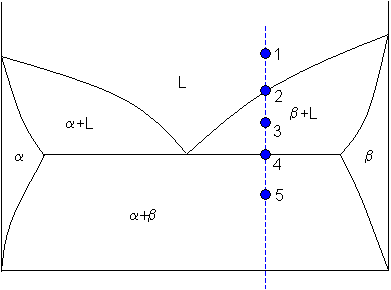
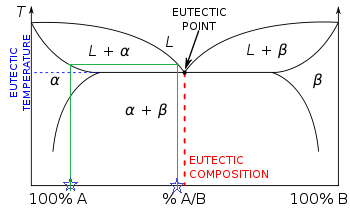
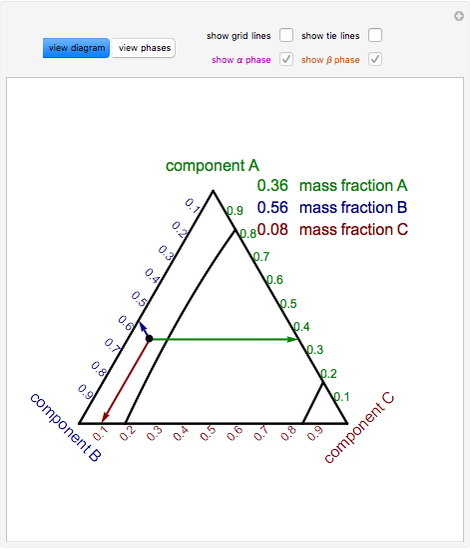
are the tie lines that connect the two equilibrium phases. As always with phase diagrams, a point in the gray area represents only a nominal composition of the whole system, but the actual phases in equilibrium are given by the ends of the tie lines on the equilibrium or coexistence curve. The lever rule also applies to these phase diagrams. The green dashed line below is an example of a tie-line. A tie-line is a horizontal (i.e., constant-temperature) line through the chosen point, which intersects the phase boundary lines on either side. Point 3. A tie-line is drawn through the point, and the lever rule is applied to identify the proportions of phases present.
There are no tie lines for the three-phase region since compositions are given by the vertices of the small triangle in the center of the diagram. In other words, compositions of each of the three-phases in equilibrium are invariant and do not depend on the overall composition.

Tie lines phase diagram
by N Bezlyepkina · Cited by 94 — Phase diagram and tie-line determination for the ternary mixture DOPC/eggSM/Cholesterol. Supporting Material. N. Bezlyepkina, R. S. Gracià†, ... 1. Locate composition and temperature in diagram 2. In two phase region draw the tie line or isotherm 3. Fraction of a phase is determined by taking the length of the tie line to the phase boundary for the other phase, and dividing by the total length of tie line The lever rule is a mechanical analogy to the mass balance calculation. If the entire length of the tie line mo is taken to represent 100% or the total weight of the two phases present at temperature T, the lever rule may be expressed mathematically as: For such phase diagram, there is one degree of freedom. So, in this case Gibb's rule converts into
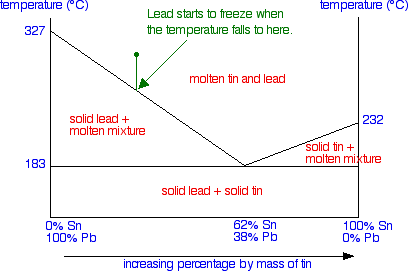
Tie lines phase diagram. Tie Lines in Phase Diagrams for Ternary Liquid Systems Most laboratory programs in physical chemistry a t the undergraduate level include an exercise in the construction of a phase diagram, on triangular coordinate paper, for a ternary liquid system. The experimental method used for this exercise is almost always a titration method in which the ... By considering tie lines and the phase diagram, it can be seen that beta has a decreasing proportion of A in it as the temperature decreases. Similarly the proportion of B in alpha decreases. Even though the alloy is now solid, the composition of the alpha and beta lamella must continue to Tie lines connect compositions of liquid and vapor phases in equilibrium. What is 3phase diagram? Three Phase Diagram: A soil mass consist of the solid particles and the voids in between them. So there is a three phase system, but when the voids are only filled with air, or only filled with water then soil becomes a two phase system. Three ... The horizontal lines are called "tie lines." The two phases at the ends of the tie lines are in equilibrium. If you follow the line for say for 12 $^\circ$ C, from the degree notation there is at first one phases, a gas phase.
196 / Phase Diagrams—Understanding the Basics. worth noting that an isopleth is a collection of ZPF lines for the various phases present. Computer programs that predict phase diagrams can give . a phase diagram in the form of ZPF lines alone. In this case, the lines are labeled instead of the regions. 10.2 The Gibbs Triangle Describes how to interpolate equilibrium tie lines on a ternary diagram. Made by faculty at the University of Colorado Boulder Department of Chemical and Bio... As Fig. 3 shows, at 1,000 psia the two-phase region is a band that stretches from the C 1 -C 10 side of the diagram to the tie line on the C 1 -C 4 side. If the pressure is increased above 1,000 psia, the liquid composition line shifts to higher methane concentrations; methane is more soluble in both C 4 and C 10 at the higher pressure (see Fig. 4).The two-phase region detaches from the C ... 1. Locate composition and temperature in diagram . 2. In two phase region draw the tie line or isotherm . 3. Fraction of a phase is determined by taking the length of the tie line to the phase boundary for the other phase, and dividing by the total length of tie line . The lever rule is a mechanical analogy to the mass balance calculation. The ...
two-phase area on the triangular diagram was small, and only three or four tie lines could be determined with con- venience. Cvclohexane omduced the converse oroblem. Tie Lines in Phase Diagrams for Ternary Liquid Systems The two-ph&e area fillei almost the whole of the'triangle, and the turbiditv end~oints were difficult. A tie line is specifically for a two-phase region, regardless of the dimension of the phase diagram (binary/ternary/quaternary). So in a 2-phase region, simply proceed as in the binary case. Coming... Phase Diagrams (Tie line, Lever Rule, Indentifcation of compositions and weight fractions in two-phase regions)Prof. Ratna Kumar Annabattula Department of Me... The tie line on a two-component solid- liquid phase diagram is an isothermal line that connects two equilibrium phases and passes through the alloy's position on the phase diagram when it is in a two-phase field, which intersects the two adjacent solubility curves. In a solid-liquid phase diagram, the solid-liquid phase boundary which is also ...
An isothermal (constant temperature) line through the alloy's position on the phase diagram when it is in a two phase field, intersecting the two adjacent solubility curves, is called a tie line (yes, that's the horizontal yellow line on the diagram). Click to see full answer Correspondingly, what is a tie line in ternary phase diagram?
I have designed a phase diagram using a turbidity titration method. The qualities of salt and solvent were identified using a binodal curve to make a phase diagram. Now I want to draw tie-lines ...
tie-lines in at least one phase diagram of interest. 2. Materials and Methods 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine(DPPC),andcholes-terol (Chol) were purchased in lyophilized form from Avanti Polar Lipids (Alabaster, AL) and used as received. Fresh stock
The tie lines determined in the two-liquid-phase coexistence region are found to be not parallel, suggesting that the dominant mechanism of lipid phase separation in this region changes with the membrane composition. We provide a phase diagram of the DOPC/eSM/Chol mixture and predict the location of the critical point.
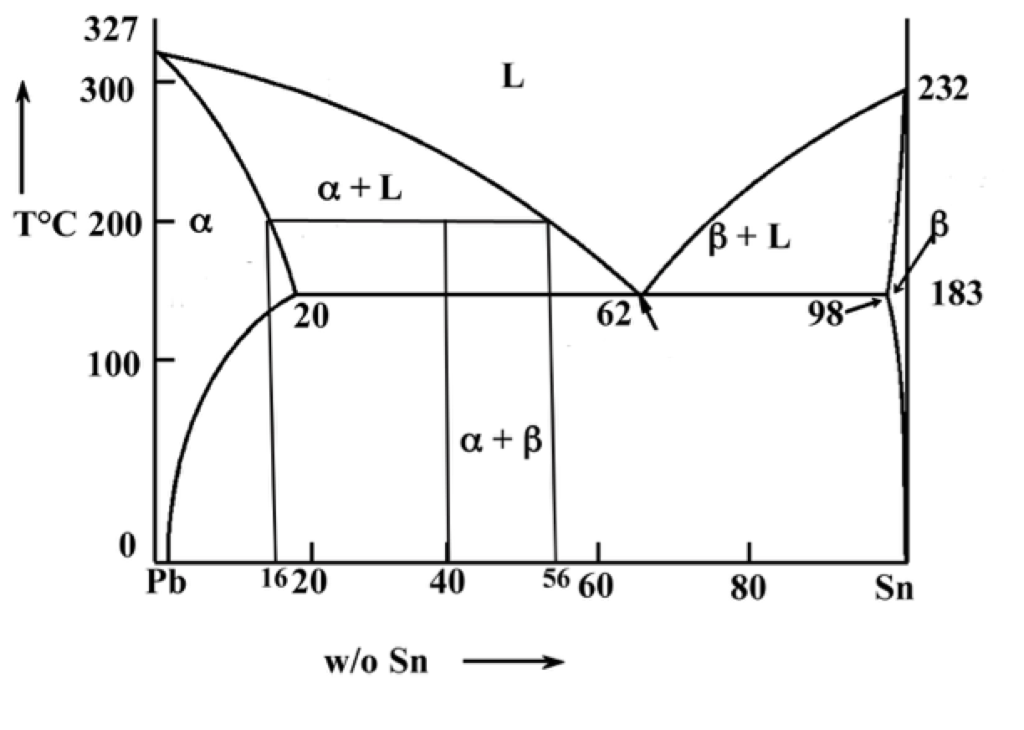
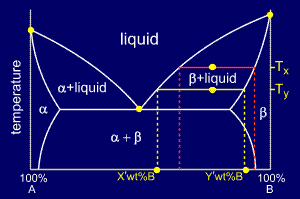
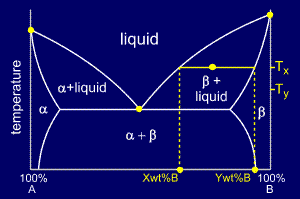
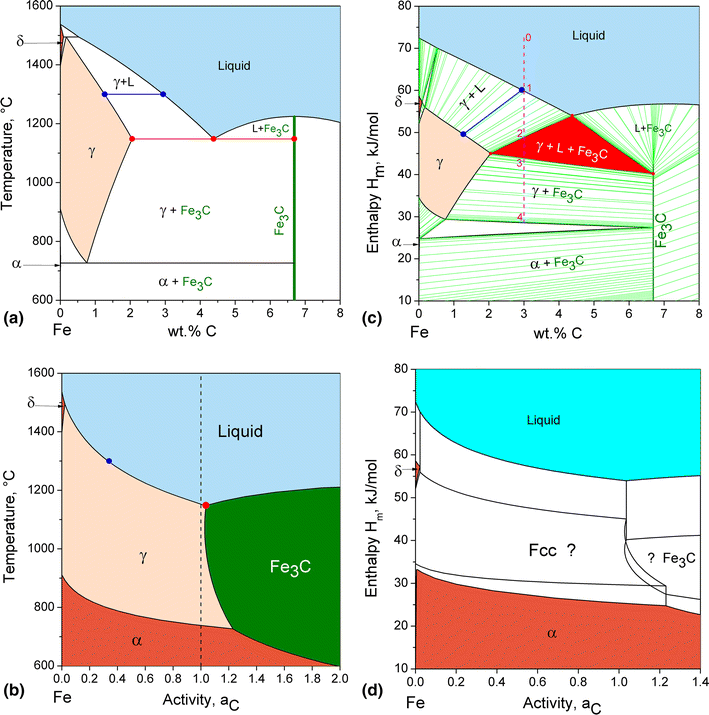
Binary phase diagrams. Before any calculations can be made, a tie line is drawn on the phase diagram to determine the mass fraction of each element; on the phase diagram to the right it is line segment LS. This tie line is drawn horizontally at the composition's temperature from one phase to another (here the liquid to the solid).
The tie lines determined in the two-liquid-phase coexistence region are found to be not parallel, suggesting that the dominant mechanism of lipid phase separation in this region changes with the membrane composition. We provide a phase diagram of the DOPC/eSM/Chol mixture and predict the location of the critical point.
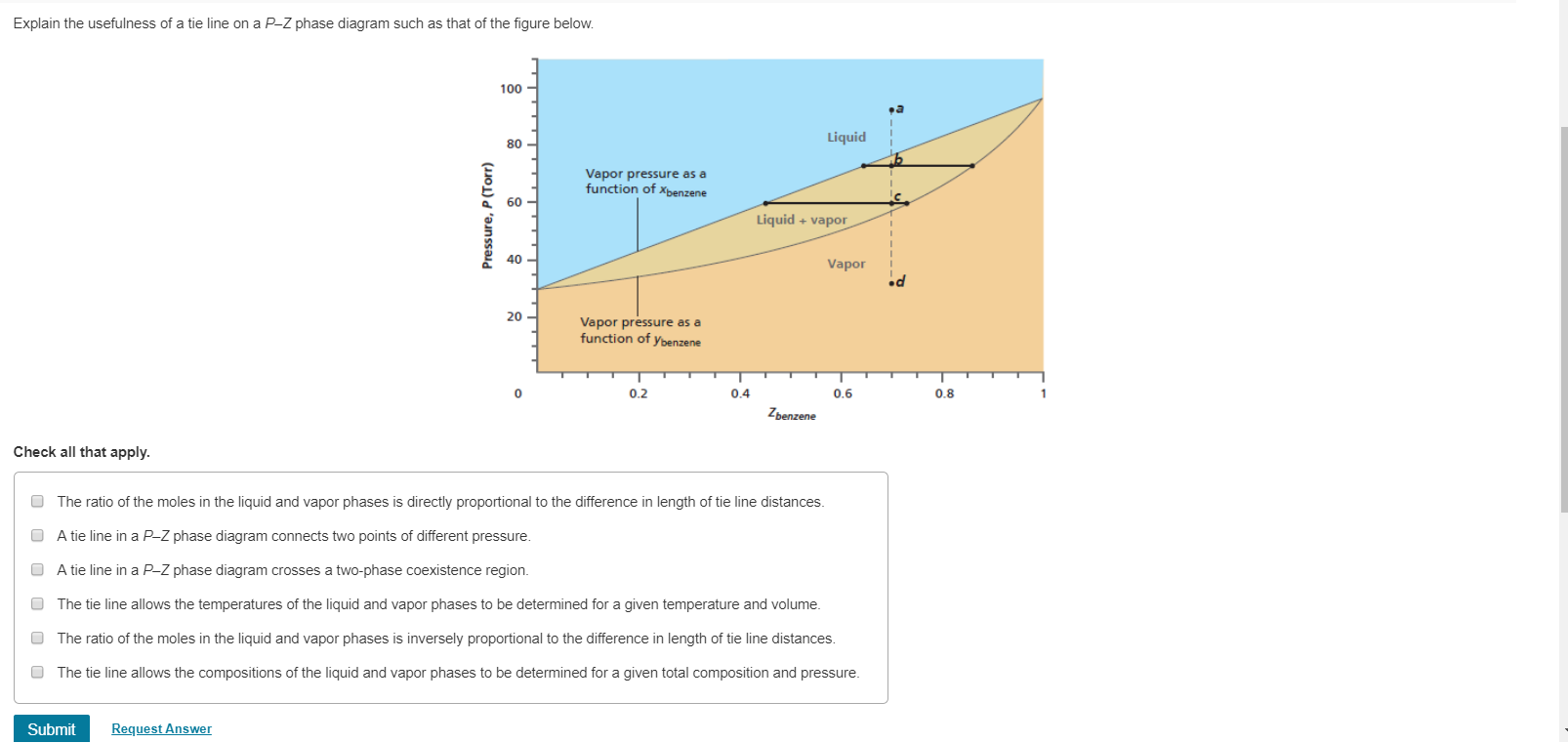
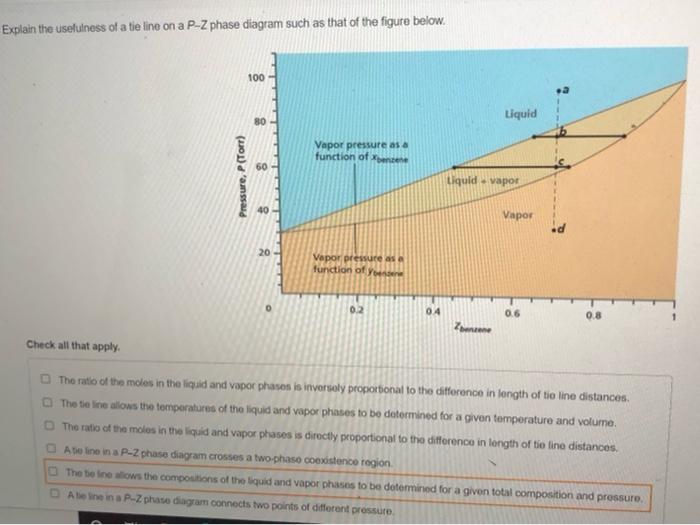
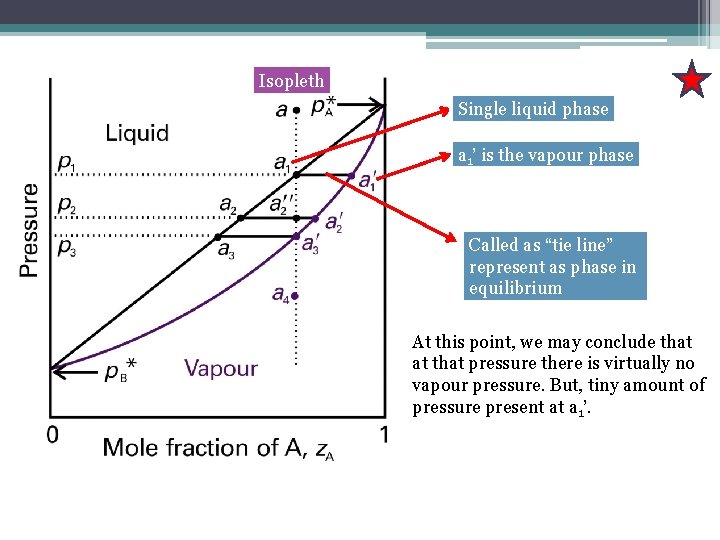
A point in the two-phase region of a phase diagram indicates not only qualitatively that the liquid and vapor are present, but represents quantitatively the relative amounts of each. To find the relative amounts of two phases a & b in equilibrium, we measure distances on the tie line, l a and l b between the two phases and use the lever rule:
An isothermal (constant temperature) line through the alloy's position on the phase diagram when it is in a two phase field, intersecting the two adjacent solubility curves, is called a tie line (yes, that's the horizontal yellow line on the diagram).. The ends of the tie lines show the compositions of the two phases that exist in equilibrium with each other at this temperature.
People also ask, what is a tie line in phase diagram? An isothermal (constant temperature) line through the alloy's position on the phase diagram when it is in a two phase field, intersecting the two adjacent solubility curves, is called a tie line (yes, that's the horizontal yellow line on the diagram). Similarly, what is a ternary system?
in 2-phase region: 1. Draw the tie line. 2. Note where the tie line intersects the liquidus and solidus lines (i.e. where the tie line crosses the phase boundaries). 3. Read off the composition at the boundaries: Liquid is composed of CL amount of Ni (31.5 wt% Ni). Solid is composed of Cαααα amount of Ni (42.5 wt% Ni).
Tie lines connect the compositions of liquid and vapor phases in equilibrium at a fixed pressure. Thus, the ternary phase diagram for CO 2 -C 4 -C 10 mixtures at any pressure is just a constant pressure (horizontal) slice through the triangular prism.
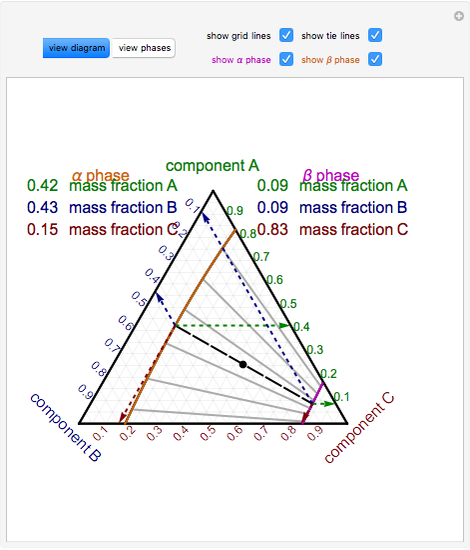
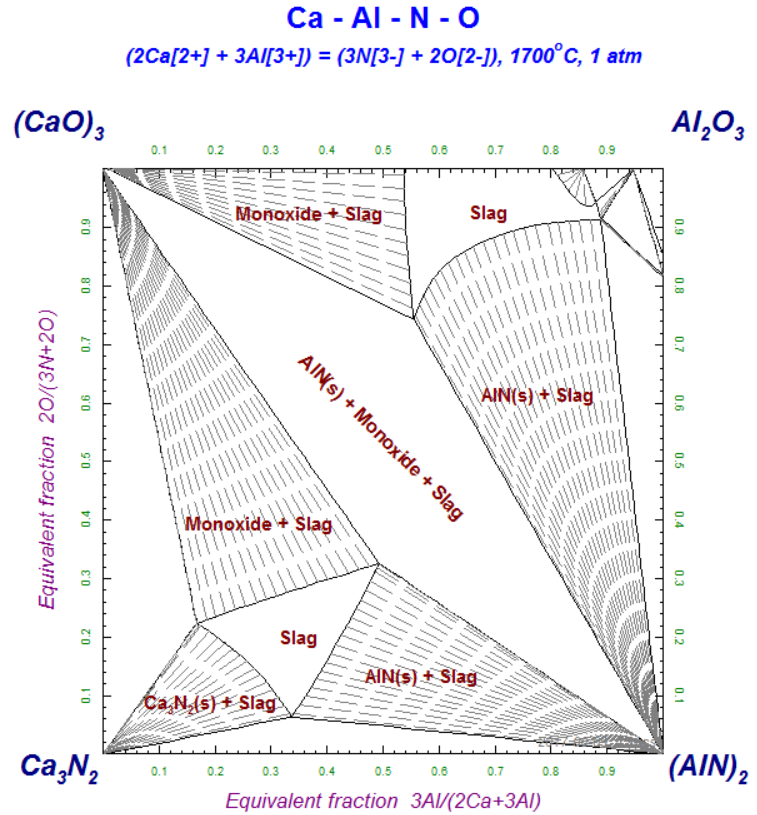
Ternary phase diagram of water/ethanoic acid/ethyl ethanoate system with the tie-lines corresponding to mixtures A, B, C, and D at T = 298.15 K under P = 101325 Pa. The results obtained in the study of liquid-liquid extraction for the water/ethyl ethanoate/ethanoic acid ternary system were summarized in Figure 3.
Solving VLE Using Raoult's Law and Iterative Method Solver. Distillation of a Two Component Mixture (Part I) Distillation of a Two Component Mixture (Part II) Gas Stripping (Henry and Raoult's Laws) Solubility Introduction. Using Solubility Diagrams for Material Balances. Interpolating Tie Lines on a Ternary Diagram.
If the entire length of the tie line mo is taken to represent 100% or the total weight of the two phases present at temperature T, the lever rule may be expressed mathematically as: For such phase diagram, there is one degree of freedom. So, in this case Gibb's rule converts into
1. Locate composition and temperature in diagram 2. In two phase region draw the tie line or isotherm 3. Fraction of a phase is determined by taking the length of the tie line to the phase boundary for the other phase, and dividing by the total length of tie line The lever rule is a mechanical analogy to the mass balance calculation.
by N Bezlyepkina · Cited by 94 — Phase diagram and tie-line determination for the ternary mixture DOPC/eggSM/Cholesterol. Supporting Material. N. Bezlyepkina, R. S. Gracià†, ...














0 Response to "39 tie lines phase diagram"
Post a Comment