38 consider the sugar-water phase diagram
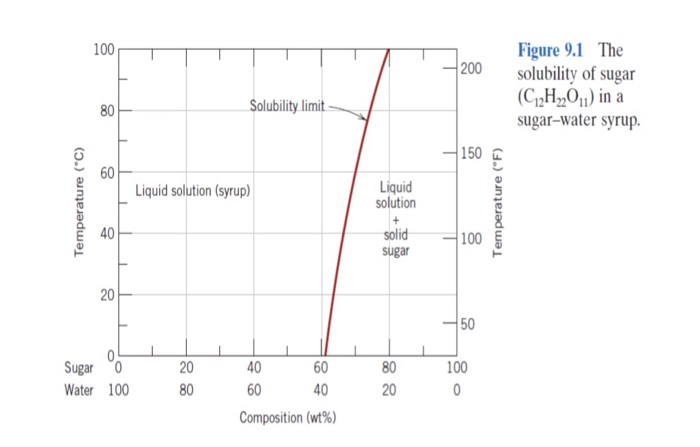
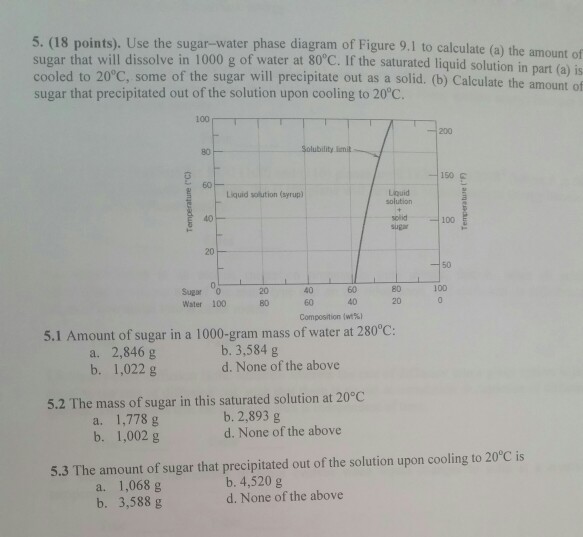
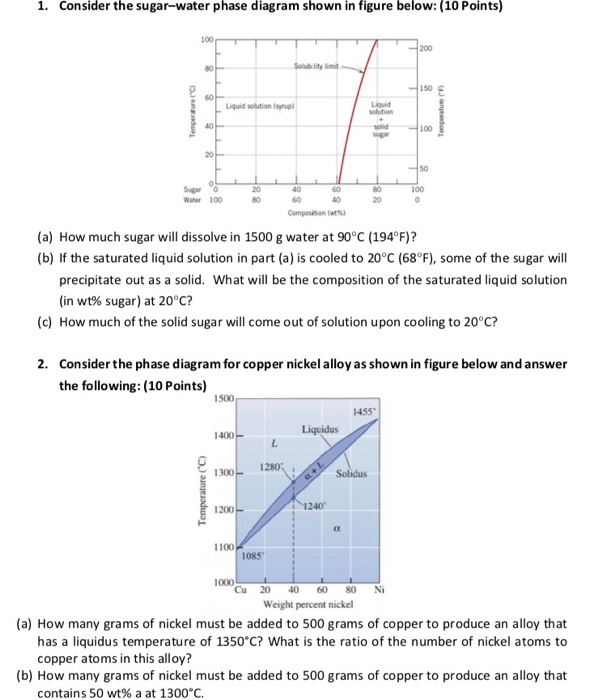
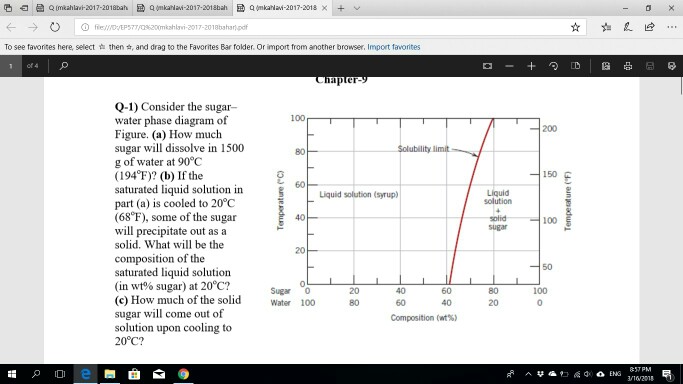
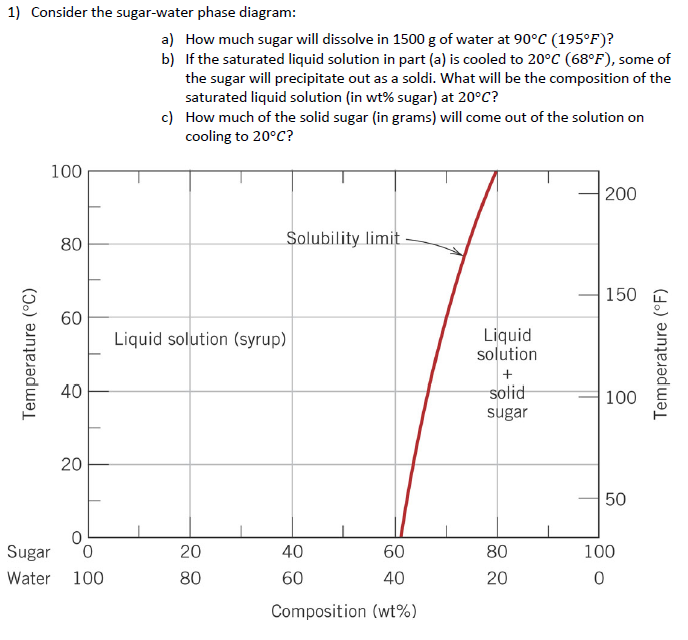
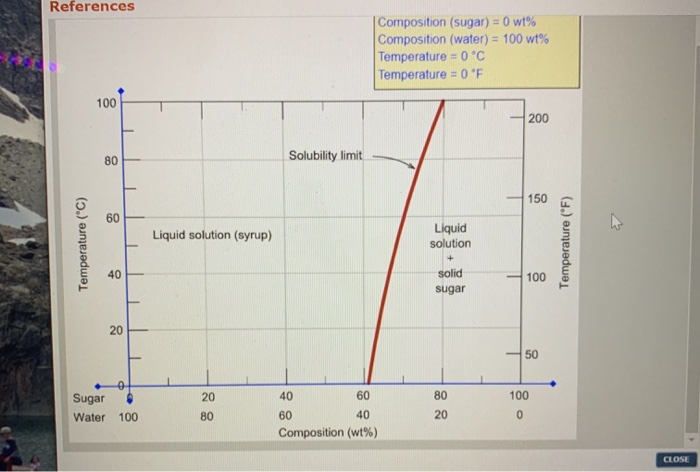
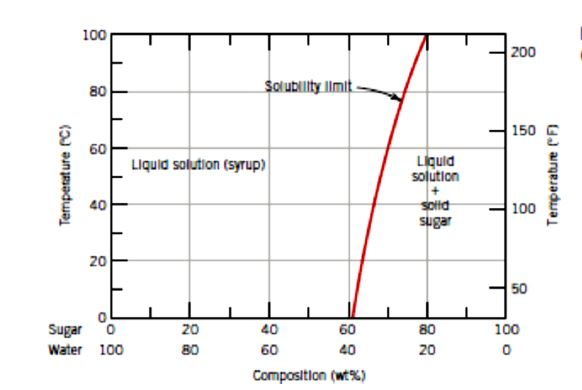
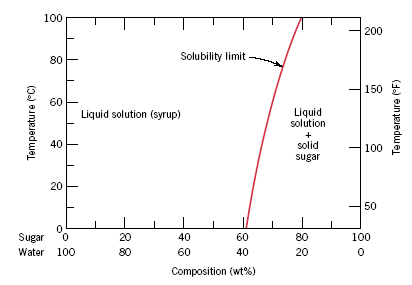
CHAPTER 9 PHASE DIAGRAMS PROBLEM SOLUTIONS Solubility Limit 9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90°C (363 K)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (293 K), some of the sugar will precipitate out as a solid. 9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90°C (194°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid. What will be the composition of the saturated liquid solution (in wt% sugar) at 20°C?
Jan 18, 2021 — 1 Answer to Consider the sugar–water phase diagram of Figure. (a) How much sugar will dissolve in 1500 g water at 90(C (194(F)?1 answer · Top answer: Solution: a) Given Data: Mass of water \(m_w=1500\:g\) Temperature \(T=90^0C\) Now from the given suger-water phase diagram the concentration of sugar ...How much of the solid sugar will come out of solution upon ...Jan 27, 20211. Consider the sugar–water phase diagram of Figure 10.1. (a ...Oct 7, 2021Consider the sugar–water phase diagram shown in Figure P8 ...Jul 5, 2021More results from www.transtutors.com

Consider the sugar-water phase diagram
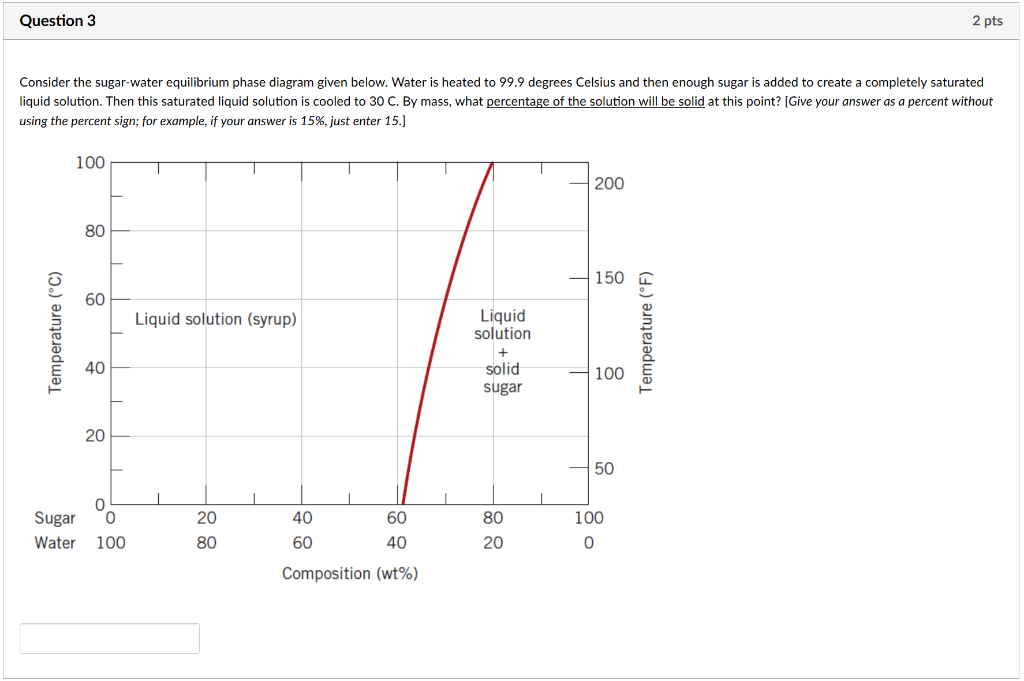
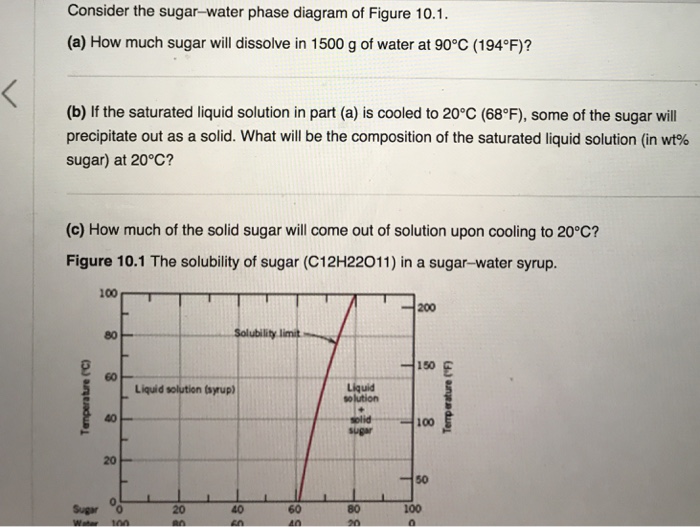
9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90 C (194 F)? (b) If the saturated liquid solution in part (a) is cooled to 20 C (68 F), some of the sugar will precipitate out as a solid. What will be the composition of the saturated liquid solution (in wt% sugar) at 20 C? Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90°C (363 K)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (293K), some of the sugar will precipitate out as a solid. What will be the composition of the saturated liquid solution (in wt% sugar) at 20°C? Q.2. Consider the sugar-water phase diagram, Animated Figure 10.1: (a) How much sugar will dissolve in 1000 g of water at 70°C? (b) If the saturated liquid solution in part (a) is cooled to 20°C, some of the sugar will precipitate out as a solid, because as seen in the figure, the composition of the saturated liquid solution drops to 64 wt% sugar.
Consider the sugar-water phase diagram. Consider the sugar-water phase diagram of _ Figure 9.1 (a) How much sugar will dissolve in 1500 g water at 90 9 (194 F)? If the saturated liquid solution in ...4 answers · Top answer: in Problem 36 were given a piece of a phase transition diagram where two solutions are said ... 8.1 Phase Diagram of Water • Field - 1 phase • Line - phase coexistence, 2 phases ... Consider the binary equilibrium phase diagram of elements A and B that are completely soluble in each other C o ... sugar) • Changing T can change # of phases: path A to B. 9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g of water at 90°C (194°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid. Download scientific diagram | The sugar/water phase diagram for the sugar/water system with boiling point line included. Prepared from data referenced in the text. from publication: Building a Low ...
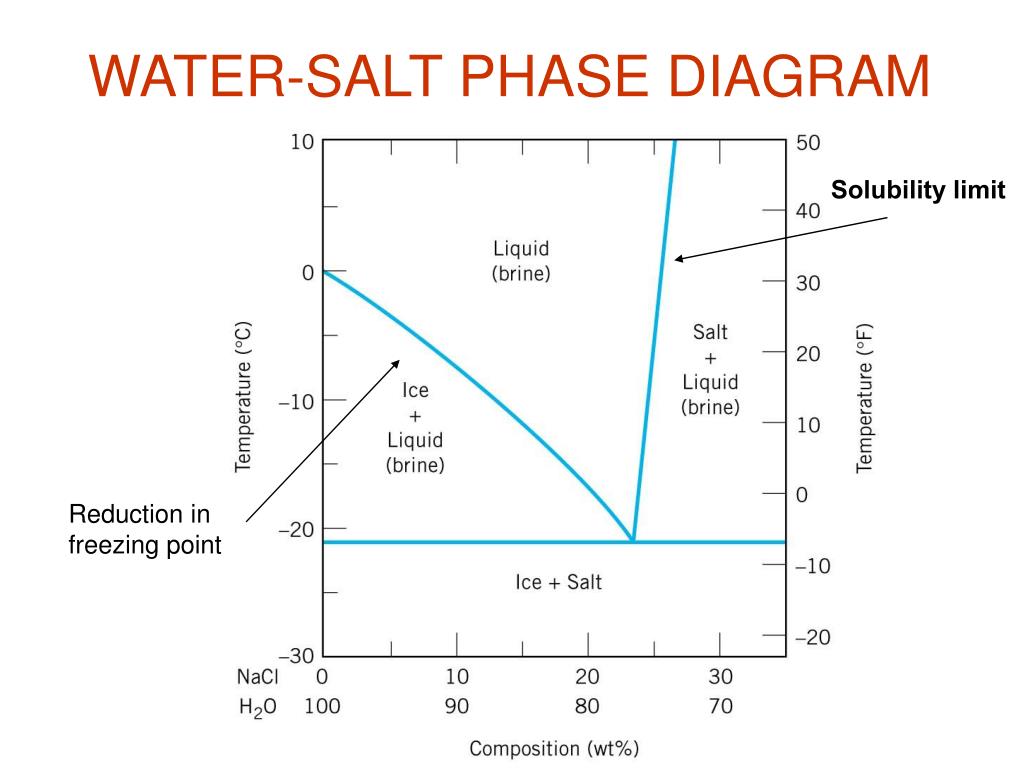
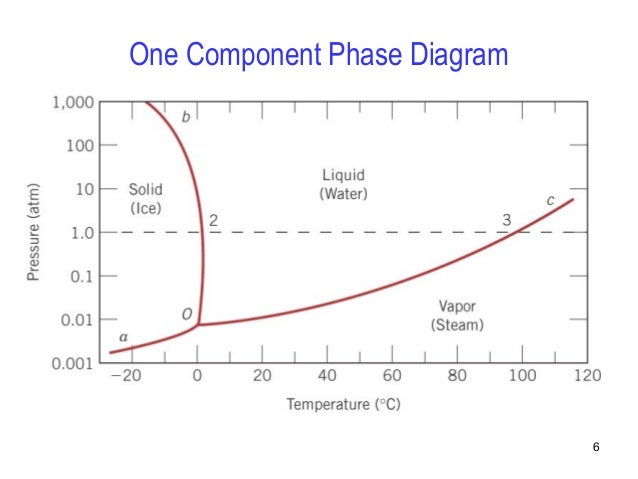
Chapter 9 1 Phase Diagrams pendium of Evaluated Constitutional Data and Phase Diagrams, Wiley, New York, 1988—1995. Fifteen volumes. QUESTIONS AND PROBLEMS 9.1 Consider the sugar—water phase diagram of REVISED PAGES EQA Handbook of Ternary Alloy Phase Diagrams, ASM International, Materials Park, OH, 1995. Ten volumes. Also on CD. Composition Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ... Question 4 & 5 (see picture for diagram): Consider the following phase diagram of water. The temperature and pressure scales are greatly reduced (and are non-linear). Point A is associated with which of the following? ... A student is preparing a sugar water solution to make rock candy. When the student continues to add sugar to the solution ... Consider a sugar-water phase diagram: (a) How much sugar will dissolve in 1000 g of water at 90{eq}^o {/eq} C? (b) If the saturated liquid solution in part (a) is cooled to 20{eq}^o {/eq} C, some ...
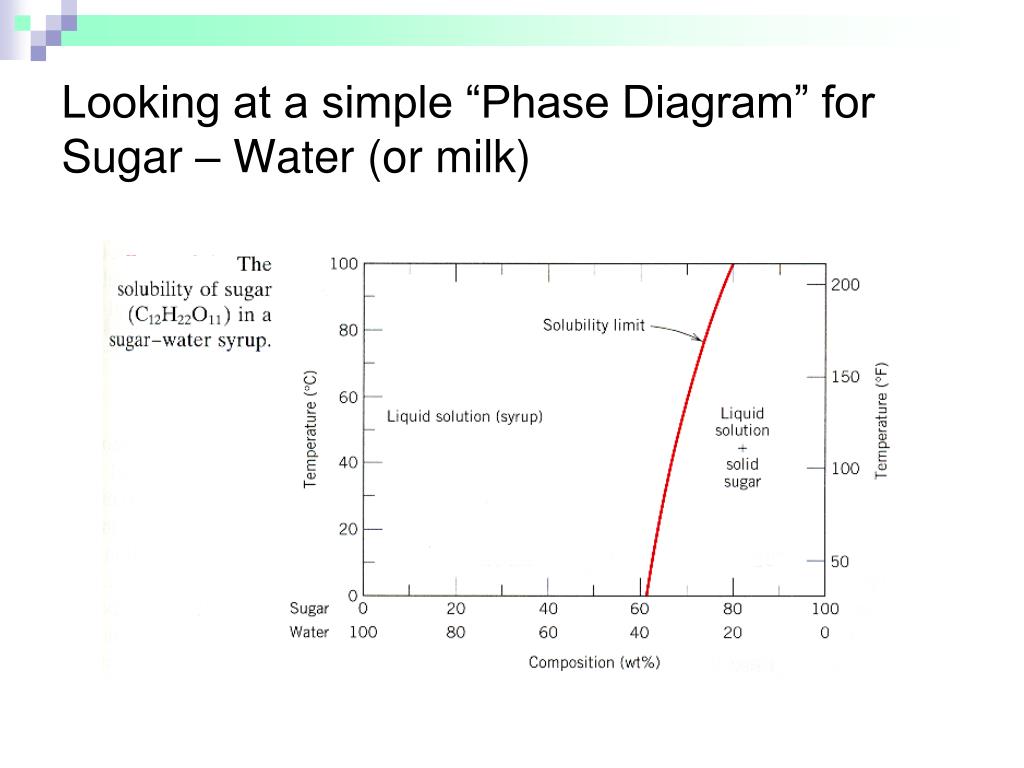
which only a single phase solution exists. Sugar/Water Phase Diagram Sugar Temperature (° C) 0 20 40 60 80 100 C = Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S (solid 20 sugar) 40 60 80 100 Water Adapted from Fig. 10.1, Callister & Rethwisch 3e. Chapter 10 - 4 Components and Phases • Components: Phase Equilibria: Solubility Limit 65 Sucrose/Water Phase Diagram Pure Sugar emperature (°C) 0 20 40 60 80 100 C o =Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S (solid 20 sugar) 4 0 6 0 8 0 10 0 Pure ater Question: What is the solubility limit at 20C? Answer: 65wt% sugar. If Co < 65wt% sugar: syrup If ... Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90°C (194°F)? Students also viewed these Thermodynamics questions. Consider the sugar-water phase diagram of Figure 9.1. solubility limit for sugar in water at 20°C? Answer: 65 wt% sugar. At 20°C, if C < 65 wt% sugar: syrup At 20°C, if C > 65 wt% sugar: syrup + sugar 65 • Solubility Limit: Maximum concentration for which only a single phase solution exists. Sugar/Water Phase Diagram S u g a r T e m p e r a t u r e (° C) 0 20 40 60 80 100 C= Composition (wt ...
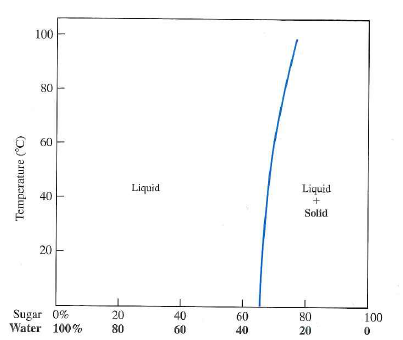
characteristics. Every pure material is considered to be a phase. Every solid, liquid, and gaseous solution may be considered a phase. In above example: sugar-water syrup solution is one phase. The solid sugar is another phase. If two or more phases present in a given system, a boundary separating the phases will exist across
Phase diagram questions. 14:440:407 ch9 Question 9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90°C (194°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid. What will be the composition of the ...
Solubility Limit 9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1000 g of water at 80°C (176°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid.
1. Problem 10.01 Consider the sugar-water phase diagram, Animated Figure 10.1 (a) How much sugar will dissolve in 1000 g of water at 90°C? g (b) If the saturated liquid solution in part (a) is cooled to 20°C, some of the sugar will precipitate out as a solid, because as seen in the figure, the composition of the saturated liquid solution drops to 64 wt% sugar.
PHASE DIAGRAMS PROBLEM SOLUTIONS Solubility Limit 10.1 Consider the sugar-water phase diagram of Figure 10.1. (a) How much sugar will dissolve in 1000 g of water at 80°C (176°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid.
cristobalite, as well as a non-crystalline phase, silica glass, and as molten silica. Every pure material is considered to be a phase, so also is every solid, liquid, and gaseous solution. For example, the sugar-water syrup solution is one phase, and solid sugar is another. 2
Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1000 g of water at 80°C (176°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid.
Sucrose/Water Phase Diagram Pure Sugar Temperature (°C) 0 20 40 60 80 100 Co=Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility LimitL (liquid) + S (solid 20 sugar) 40 60 80 100 Pure Water Adapted from Fig. 9.1, Callister 7e. Chapter 9 - 3 • Components : The elements or compounds which are present in the mixture (e.g., Al and Cu)
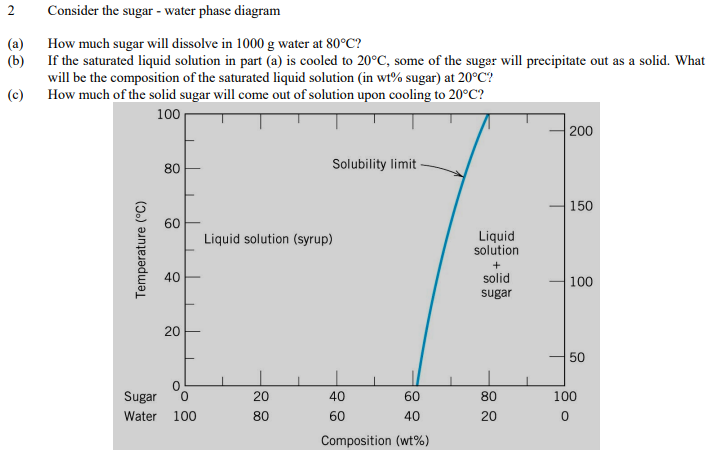
2 Consider the sugar - water phase diagram (a) (b) How much sugar will dissolve in 1000 g water at 80°C? If the saturated liquid solution in part (a) is cooled to 20°C, some of the sugar will precipitate out as a solid.
PHASE DIAGRAMS PROBLEM SOLUTIONS Solubility Limit 9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90 °C (194 °F)? (b) If the saturated liquid solution in part (a) is cooled to 20 °C (68 °F), some of the sugar will precipitate out as a solid.
Consider the sugar-water phase diagram below, (a) How much sugar will dissolve in 1000 g of water at 80°C (176°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid.
Determining the State of Water Using the phase diagram for water given in , determine the state of water at the following temperatures and pressures: (a) −10 °C and 50 kPa (b) 25 °C and 90 kPa (c) 50 °C and 40 kPa (d) 80 °C and 5 kPa (e) −10 °C and 0.3 kPa (f) 50 °C and 0.3 kPa. Solution
Sugar/Water Phase Diagram Sugar Temperature (o C) 0 20 40 60 80 100 C = Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S (solid 20 sugar) 40 60 80 100 Water Adapted from Fig. 11.1, Callister & Rethwisch 9e. ... Consider C0= 35 wt% Ni D T D
Consider the sugar-water phase diagram, Animated Figure 10.1: (a) How much sugar will dissolve in 1000 g of water at 70°C? (b) If the saturated liquid solution in part (a) is cooled to 20°C, some of the sugar will precipitate out as a solid, because as seen in the figure, the composition of the saturated liquid solution drops to 64 wt% sugar.

I’m always mesmerized by the dance between light and water. I took this photo while waiting poolside for the models to set up during a styled shoot in Maui.
Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90°C (363 K)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (293K), some of the sugar will precipitate out as a solid. What will be the composition of the saturated liquid solution (in wt% sugar) at 20°C? Q.2.
9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90 C (194 F)? (b) If the saturated liquid solution in part (a) is cooled to 20 C (68 F), some of the sugar will precipitate out as a solid. What will be the composition of the saturated liquid solution (in wt% sugar) at 20 C?
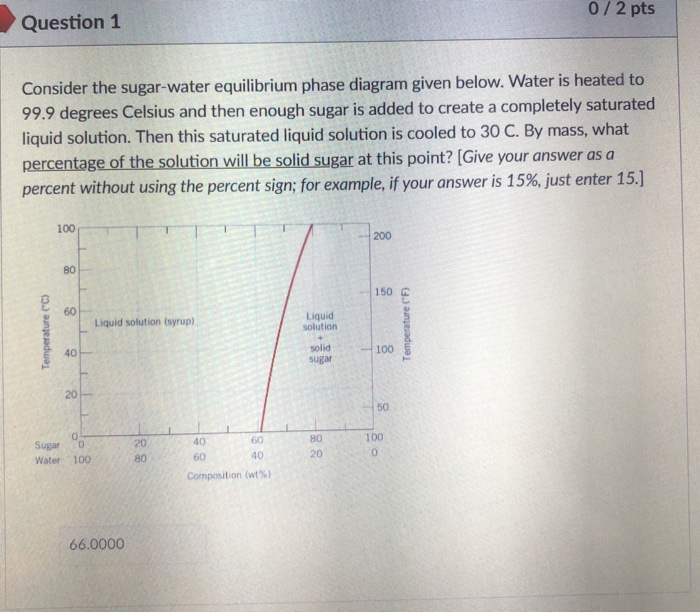











0 Response to "38 consider the sugar-water phase diagram"
Post a Comment