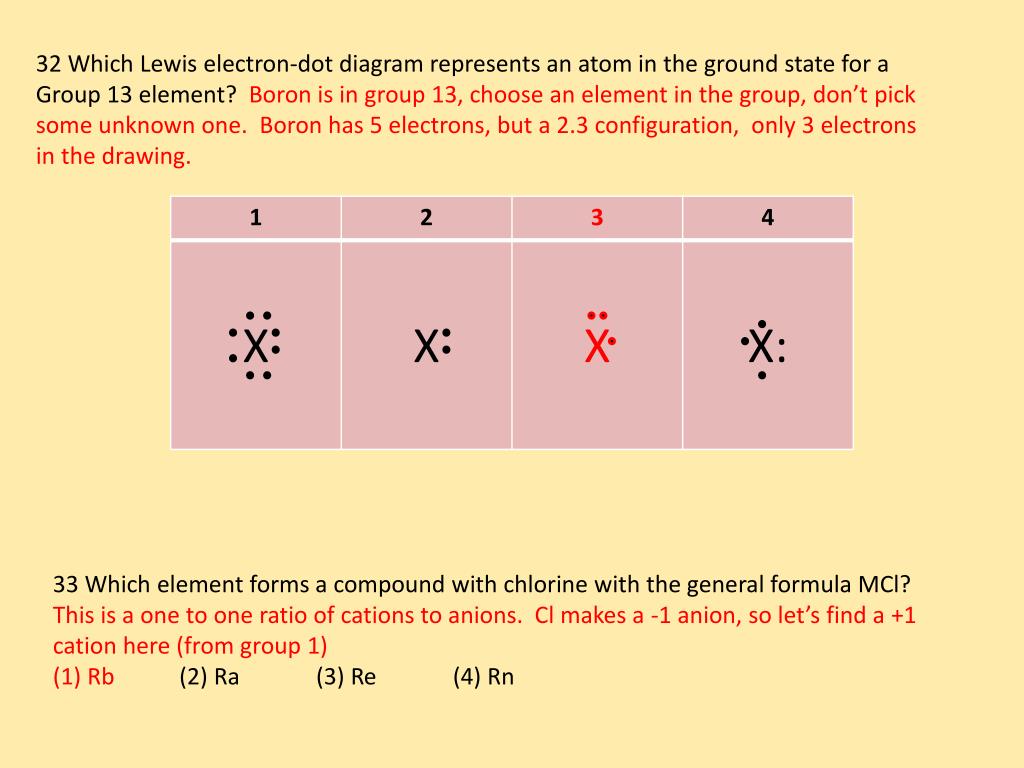
40 how many dots belong in the electron dot diagram of a boron (b) atom? three five eight thirteen
The diagram shows an electron shell model of a sodium atom. How would the model change as the atom forms bonds? The third shell would be empty so that the eight ... Rating: 5 · 2 reviews
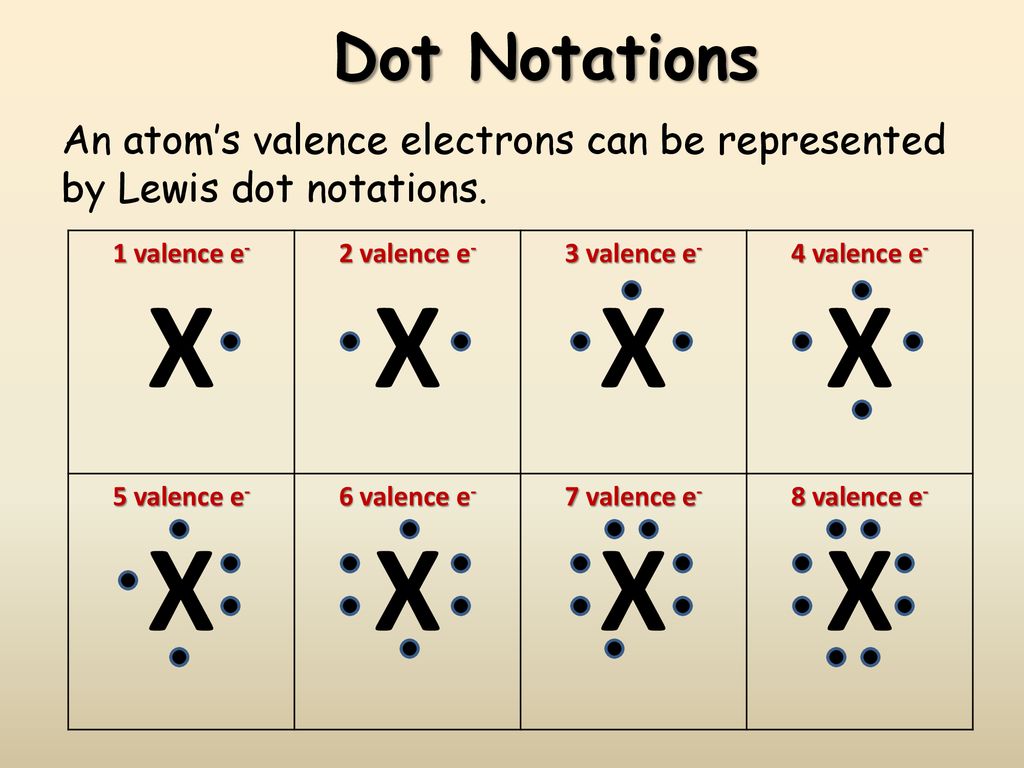
What is this electrons dot diagram is made up of? Electrons is the atom's outmost energy level are the elections that are important in chemical bond and reactions. And these electrons are only represented in the form of an electrons dot diagram.
How many electrons does each chlorine atom share? A. 1 B. 2 C. 3 D. 4 7. __B__In question number 6 above, how many valence electrons are in each bonded chlorine atom? C__Which of the following describes the process of aluminum phosphide decomposing into aluminum and phosphorus?

How many dots belong in the electron dot diagram of a boron (b) atom? three five eight thirteen
The atomic number is the number of protons in an atom of an element. Atoms must have equal numbers of protons and electrons. In our example, an atom of krypton must contain 36 electrons since it contains 36 protons.
The previous model of the atom, the Thomson atomic model, or the "plum pudding" model, in which In Bohr's model the orbits of the electrons were explained by quantum mechanics. Most of this planetary atom was open space and offered no resistance to the passage of the alpha particles.
atom: The smallest possible amount of matter which still retains its identity as a chemical element, consisting of a nucleus surrounded by electrons. proton: Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an element.
How many dots belong in the electron dot diagram of a boron (b) atom? three five eight thirteen.
Most of the rest of the atom was simply empty space. Bohr's suggestion of stable energy levels addressed the problem of electrons spiralling into the nucleus to an extent, but not entirely. At any rate, the atom gives us a great example of how scientific models can change over time, and shows...
1. The Mass Spectrometer In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that The three essential functions of a mass spectrometer, and the associated components, are: 1. A small sample is ionized, usually to cations by loss of an...
A Lewis dot diagram shows a reaction. Two chlorine symbols, each surrounded by seven. The Lewis structure indicates that each Cl atom has three pairs of ...
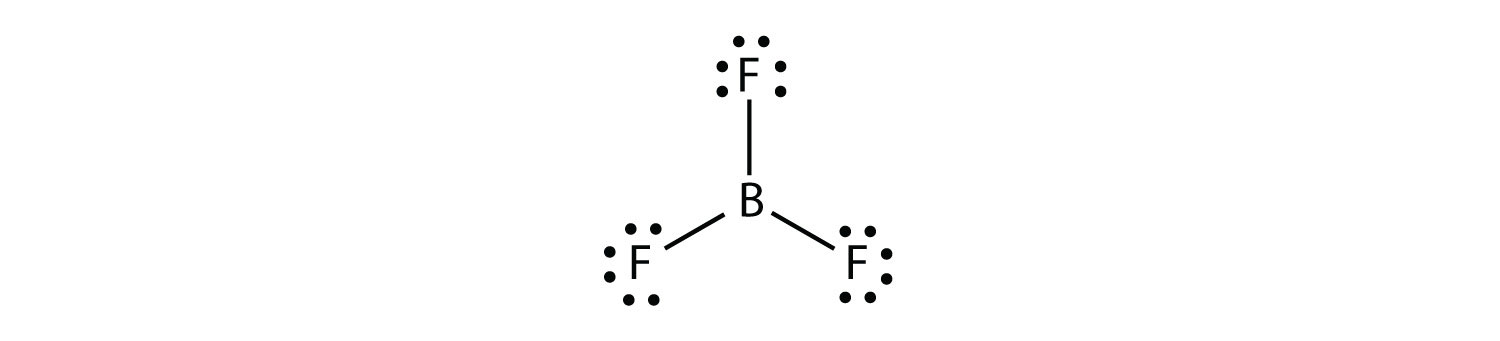
Lewis dot structure is the structure of an element or molecule, and total valence electrons are as dots to represent the bond pairs and lone pairs. Boron has an elemental symbol as "B," and the electronic configuration counts as 2,3, its atomic number 5. Hence, it has three electrons in the valence shell...
Find the required count of electrons needed to make the atoms complete. After then, define the number of bonds in the given molecule. Lewis Dot notation is a way of describing the outer shell (also called the valence shell) of an atom's electrons. Dots are drawn around the elements symbol...
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule.
The mass of elements in the middle of the table ( Periods 4 to 7) are called the transition metals. Their electron structures are more complex. Scientists initially thought that the electrons whirled around the atom's nucleus in concentric circles with the valence electron in the outer most shell.
7. The electrons at the outermost energy level 8. It is composed of the symbol of the element and dots which represent the number of valence electrons of an atom that can easily be determined through the family/group number in the Periodic Table of elements.
The number of protons in an atom is unique to each element. For example, carbon atoms have six protons, hydrogen atoms The next scientist to further modify and advance the atomic model was Rutherford, who studied under Thomson, according to the chemistry department at Purdue University.
Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. As shown in Table 1, the s subshell has one lobe, the p subshell has three lobes How many electrons can the p orbital hold? Determine the number of angular and radial nodes of a...
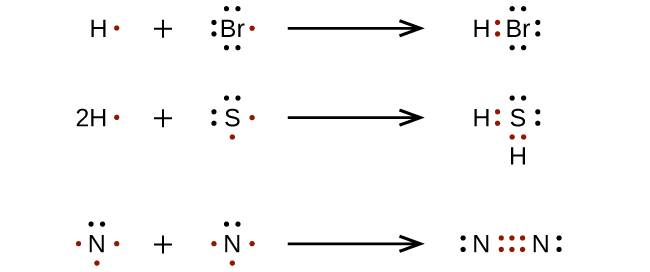
In the formation of a simple covalent bond, each atom supplies one electron to the bond - but that doesn't have to be the case. Although the electrons are shown differently in the diagram, there is no difference between them in The dots-and-crosses diagram shows only the outer electrons.
The electron configuration of an atom describes the orbitals occupied by electrons on the atom. The basis of this prediction is a rule known as the aufbau principle, which assumes that electrons are added to an atom, one at a time, starting with the lowest energy orbital, until all of the electrons...
Jul 16, 2021 · 1 answerThe answer is five dots. sorry i meant three dots not five ...
Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
6. Find the least energy of an electron moving in one-dimensional potential box (infinite height) of width 0.05nm. 7. A quantum particle confined to one-dimensional box of width 'a' is known to be in its first excited state.
In the second, third, fourth, fifth, and sixth squares is a pair each of upwards and downwards pointing arrows. Based on the diagram, what values can be assigned to the magnetic quantum number for the electrons in the atom?
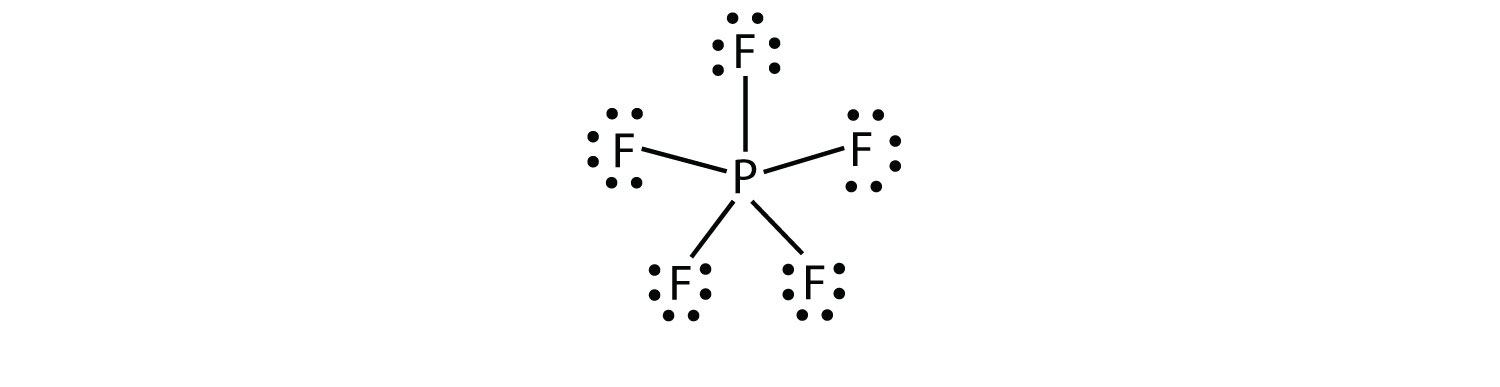
Lewis-dot diagrams of the atoms in row 2 of the periodic table are shown below: ... boron (B), and aluminum (Al) can have fewer than eight electrons around ...
The shape of the periodic table and the placement of an element in a particular group or period is derived from the electronic structure of the atoms The quantum mechanical description of an atom suggests that the electrons have a complex, but precise organization surrounding the atomic nucleus.
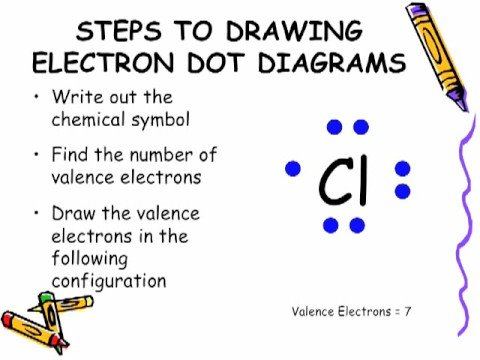
Lewis Dot structure/electron dot diagram. 1. draw chemical symbol 2. determine number of valence electrons by atom's group number 3. skip middle when counting group and drop 1 infront of groups 13-18 4. draw the valence electrons around chemical symbols.
It is known that dots present around the symbol of an element denotes the number of valence electrons present in the element. So, its electronic distribution is as 2, 8, 8, 2. Hence, it means that an atom of calcium contains 2 valence electrons and in order to attain stability it readily loses those...
Valance electrons are the electrons in the outermost layer of the Bohr model of an atom. Five protons and 6 electrons would give an atom a negative charge, as the number of electrons are Find the Number of Neutrons in an Atom. How to. Write Electron Configurations for Atoms of Any...
eight. None of Substance B is visible in the second beaker. Which of the following statements is true? Four students presented different analogies to describe the formation of an ionic bond. Which statement correctly identifies the more effective technology for predicting floods?
Atoms are very small pieces of matter. There are many different types of atoms, each with its own name, mass and size. These different types of atoms are called chemical elements. The chemical elements are organized on the periodic table.
The story of how the periodic table was discovered and developed into the scientific icon we know "The chemistry of an atom depends only on the number of electrons, which equals the number of Most elements in Group 3 lose three electrons to form 3+ ions. Boron, however, shows little...
Oct 15, 2021 — How many dots belong in the electron dot diagram of a boron (B) atom? three five eight thirteen. BTW ITS SCEINCE.
The electrons in an atom are attracted to the protons in the nucleus by the electromagnetic force. The masses of all 6 flavors of quarks, with a proton and electron (red dot) shown at the bottom left The actual mass of an atom at rest is very difficult to measure, as even the most massive of atoms...
In a Lewis electron-dot diagram of an atom shows bonding between different atoms or in a molecule. It also represents lone pairs of electrons that are present over a molecule. In this diagram, electrons in the outermost shell are represented with the help of dots around the symbol of atom or molecule.
Draw electron dot diagrams for elements. An electron dot diagram is like a football diagram. How do we show electrons in atoms? Diagrams contain a lot of ...
5 hours ago Electron dot diagram of a Magnesium atom Electron dot diagram also called lewis structure which represents the valence electrons of 2. Explain how the electron dot diagram is similar for families in the periodic table. 3. Draw an electron dot diagram showing the formation of...
Aug 27, 2020 · 2 answersAnswer: Three dots belong in the electron dot diagram of a Boron.
Next PostNext In not more than three sentences, describe the electron arrangement responsible for bonding in solid SrCl2. One way automation can lead to the creation of new jobs is by a) producing products quicker, thus shortening the work week. b) reducing the price of a product, thus increasing...
















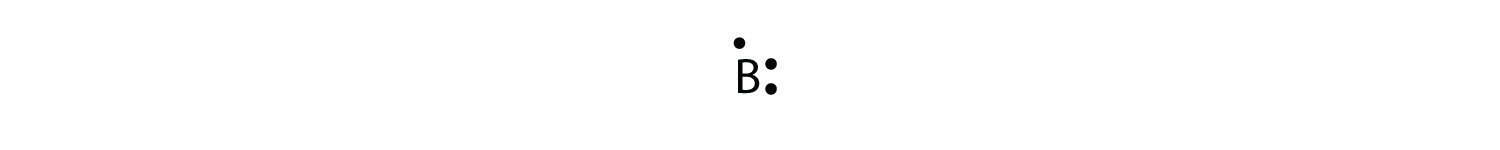





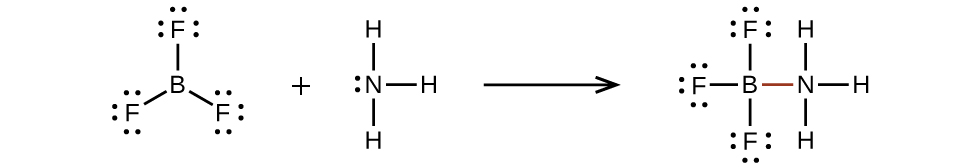


0 Response to "40 how many dots belong in the electron dot diagram of a boron (b) atom? three five eight thirteen"
Post a Comment