38 show the orbital filling diagram for n nitrogen
Orbital Filling Diagram s. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbital s are shown as circles (or squares) and orbital s within a sublevel are drawn next to each o the r horizontally. In NH4+, nitrogen and the 4 hydrogen atoms make 4 sigma bonds, out of which 3 are covalent bonds and the fourth one is a dative bond. The NH4+ ion has no pi bonds. As a result, all four electrons contained in the atomic orbitals in the outermost shell of the nitrogen atom can participate in hybridization, making it SP3.
The proper way to perform a three-point-turn (y-turn) maneuver. The 3-point turn (three-point turn), otherwise known as the three-point y-turn or simply a y-turn, is an extremely critical part of the Wisconsin road test (and also in most other states) because it is a dangerous maneuver and can have serious consequences if not properly executed.

Show the orbital filling diagram for n nitrogen
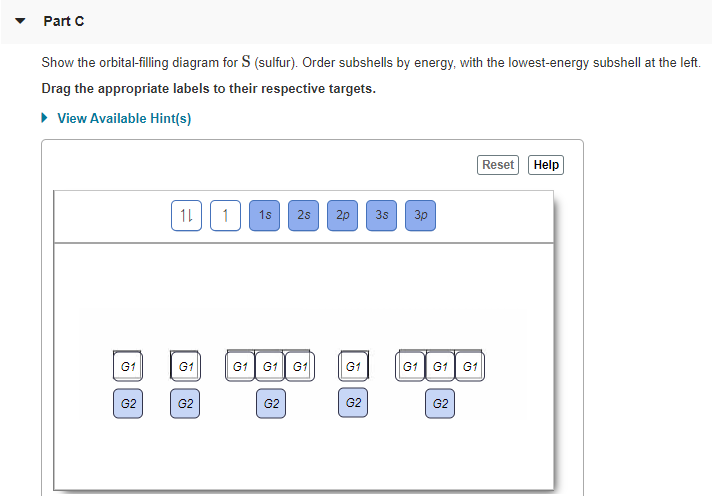
Draw an orbital-filling diagram for the element with Z = 25, showing the individual electrons in the outermost subshell as up arrows, down arrows, or 0. How many partially filled orbitals does this... Double and triple bonds. What is the electron configuration and orbital diagram of. Box spin diagram of outer electron orbitals for the electron configuration of the 15 Phosphorus P 1s22s22p63s23p3 Ne3s 3p P pblock Gp5 Build the orbital diagram for the ion most likely formed by phosphorus. The rules for orbital filling diagrams. Energy 0 1 1 x 5. Orbital Filling Diagram for Sulfur. what is the orbital diagram for sulfur the orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each the arrows solved show the orbital filling diagram for s sulfur st answer to show the orbital filling diagram for s sulfur stack the sub shells in order of energy with the ...
Show the orbital filling diagram for n nitrogen. 39 private equity fund structure diagram. Private Equity Fund structure - Master-Feeder Fund A Feeder Fund is an investment vehicle that consists in the pooling of capital commitments from investors. A Feeder Fund invests, or feeds this capital, into an umbrella fund, often called a Master Fund (Main), which directs and oversees all ... For elements 1-36, there are two exceptions to the filling order as predicted from the periodic table. Draw the atomic orbital diagrams for the two... Solved • Oct 15, 2018 Draw an orbital diagram for scandium Show the orbital-filling diagram for N (nitrogen). Get the detailed answer: Depict the electron configuration of boron (B) and scandium (Sc) using an orbital box diagram. For the diagram you start with the 1 s orbital and then 2s, 2p, and so on. Naim was happy, Naim felt safe, he crawled through a duct between decks and found the issue Mick had chirped at him, with his deft claws he unhooked a data cable unwound the fixing piece and carefully stripped back the clear wires, Naim did not know why these wires were clear, some had liquid in them others seemed to be filled with light. It didn't matter to him, as long as the Goddess was happy so was Naim, as he worked, fitting each strand into a connector. It amused him that he could recogni...
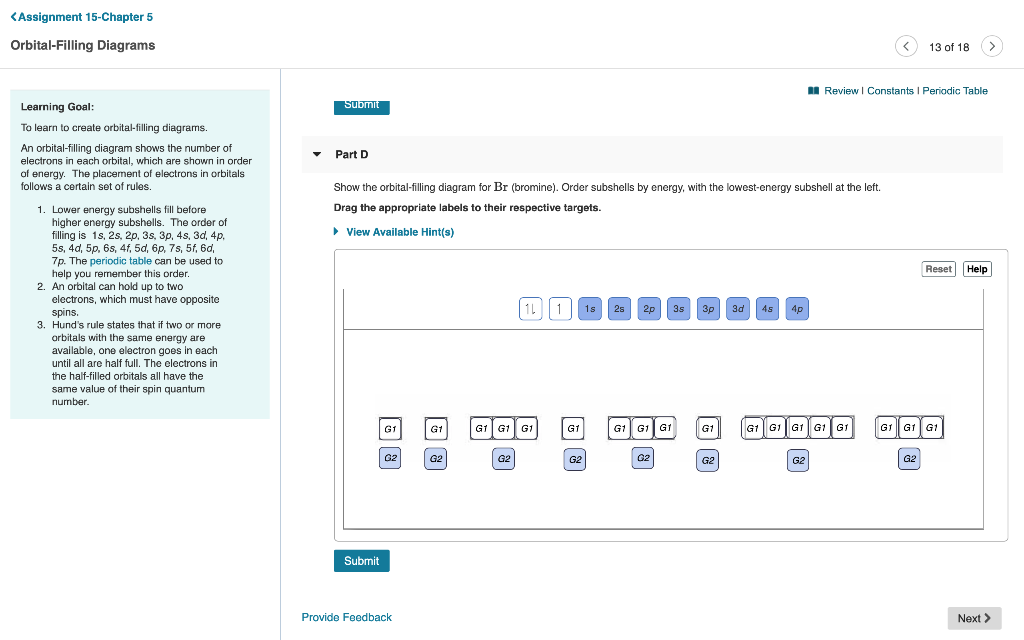
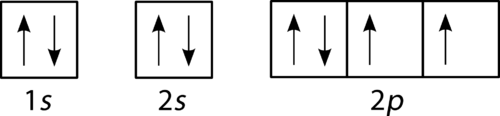
Nitrogen is the chemical element with the symbol N and atomic number 7. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772. Although Carl Wilhelm Scheele and Henry Cavendish had independently done so at about the same time, Rutherford is generally accorded the credit because his work was published first. The name nitrogène was suggested by French chemist Jean ... Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for S (sulfur). Identify the specific element that corresponds to the following electron configuration: The a to m 280X is an iso to pe of fflJn p. neon By signing up, you'll get thousands of Aug 28, 2016 · Show the orbital filling diagram for n nitrogen. If you want to learn how to draw orbital filling diagram s, you need to follow the se handy rules. Q. Part B. Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left.To learn to creat... Solved • Jul 2, 2020
The superconducting phase diagram for a quasi-two-dimensional organic superconductor, κ-(BEDT-TTF)2Cu[N(CN)2]Br, was studied using pulsed magnetic field penetration depth measurements under rotating magnetic fields. At low temperatures, Hc2 was abruptly suppressed even by small tilts of the applied fields owing to the orbital pair-breaking effect. 41 entity relationship diagram business rules. A business rule is statement that imposes some form of constraint on a specific aspect of the database, such as the elements within a field specification for a particular field or the characteristics of a given relationship. You base a business rule on the way the organization perceives and uses ... Nov 16, 2021 · The transition metal–nitrogen–carbon (M–N–C) materials and metal-organic frameworks (MOFs) are demonstrated with superior activity for electrochemical CO 2 RR, which have been described in ... Orbital Filling Element 1s 2s 2p x 2p y 2p z 3s Configuration Electron Configuration s Electron H He Li C N O F Ne Na 1s1 1s 22s 2p63s1 1s22s22p6 1s 22s 2p5 1s 22s 2p4 1s22s22p3 1s22s22p2 1s22s1 1s2 NOT CORRECT Violates Hund's Rule 2 6 1 2 6 2 2 4 2 3 2 Answer to: Use the orbital-filling diagram to show the electron configuration of helium, He. Use the buttons at the top of the tool to add ...
Nov 23, 2021 · Which of the following is the correct orbital diagram for a nitrogen (n) atom_ Which of the following is the correct orbital diagram for a nitrogen (n) atom_ ...
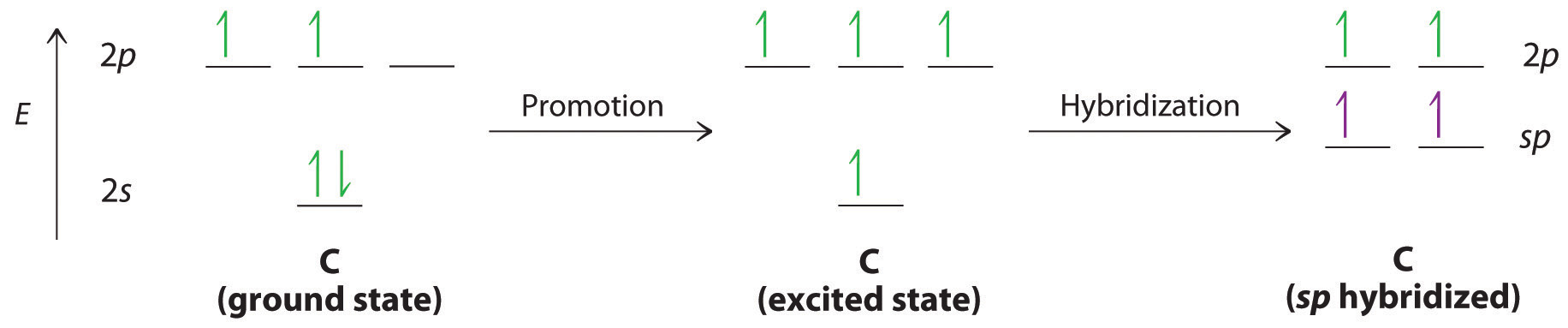
Draw the energy diagram for the orbitals of sp 3 hybridzied carbon and nitrogen. Then fill in the correct number of electron. 3. Indicate the hybridization of oxygen in each molecule. 4. Which nitrogen atom(s) is/are sp 3 hybridized. 5. Describe the bonding scheme of CH 4. Answers: 1. a and b. 2. Just like the energy diagram in fig.3.
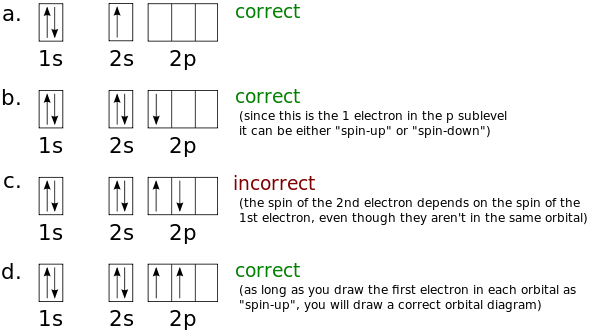
Apr 22, 2021 · To draw the orbital diagram, begin with the following observations: the first two electrons will pair up in the 1s orbital; the next two electrons will pair up in the 2s orbital. That leaves 4 electrons, which must be placed in the 2p orbitals. According to Hund’s rule, all orbitals will be singly occupied before any is doubly occupied.
The reason behind the name given to Lewis is that it was firstly used by Gilbert N. Lewis. Its main purpose is to tell the total number of Carbon Electron configurations (valence) in the atom, one more reason to use the dot diagram is that it provides us with the notations too.
help Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest- ...1 answer · 1 vote: Here is the solution of your question. If you have any doubt or need any clarification please comment in comment box and will definitely resolve your ...
Problem: Part B. Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left.1 answer · Top answer: An orbital filling diagram is a graphical representation of an electron configuration.Electron configuration is written in a way that electrons fill ...
Sep 01, 2021 · Bohr Rutherford Diagram For Nitrogen. Bohr diagrams show electrons orbiting the nucleus of an atom In the Bohr model, electrons are pictured as traveling in circles at different shells, These energy levels are designated by a number and the symbol “n. Bohr atomic model of a nitrogen atom.
Here, one sp orbital of C fuses with 1s orbital of H. And the other sp orbital of C fuses with one of the p orbitals of Nitrogen. The px orbitals of both C and N form sigma bonds while the Py and Pz orbitals form perpendicular Pi bonds. Polarity of HCN. Now let us look at whether the compound is polar or nonpolar in nature.
Since 1s. Use orbital filling diagrams to describe the locations of electrons in an atom. Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p.Show transcribed image text Show the orbital-filling diagram for N. Nitrogen is the seventh element with a total of 7 electrons.
15 May 2019 · 2 answers The line can be name d by ei the r of the po in ts in it. P or Q The plane is name d by any po in ts in it. R, S, P or Q. 1 Jul 2020 · 1 answerAnswer: The line is AB and the plane is ABD, the first option is the correct one.Step-by-step explanation:Ok, first some def in itions. Name the line and plane shown in the diagram. Postulate in geometry rules that are accepted ...
part A;Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to ...
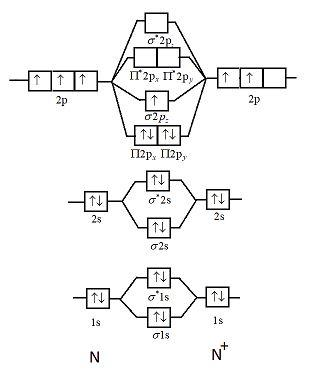
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Here is the full molecular orbital diagram for N 2.
Nitrogen is the seventh element of the periodic table with a total of 7 electrons. When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next two electrons for Nitrogen (N) go in the 2s orbital. The three electrons that are remained will go in the 2p orbital.
Molecular orbital diagram practice worksheet
Vacuum diagram 2003 ford f150 54l v8 cars trucks. Here is a diagram of the vacuum system so you can see what. This is a image galleries about f150 2003 vacuum hose diagramyou can also find other images like wiring diagram parts diagram replacement parts electrical diagram repair manuals engine diagram engine scheme wiring harness fuse box.
If X= {1, 2, 3}, if n represents any member of X, write the following sets containing all numbers represented by asked Feb 16, 2018 in Class XI Maths by vijay Expert ( 7.9k points) sets
If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital.
Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau... Orbital diagram of Nitrogen (N) 8. Orbital diagram of Oxygen (O) 9. Orbital diagram of Fluorine (F) 10. Orbital diagram of Neon (Ne) 11. Orbital diagram of Sodium (Na) An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom.
The process decomposition diagram (often called a decomp) explains the breakdown of processes within a project or business area or functional area. Functional decomposition diagram s . The purpose of the functional decomposition diagram is to show on a single page the capabilities of an organization that are relevant to the consideration of an architecture. By examining the capabilities of an ...
Exercise 221 Draw an orbital diagram for nitrogen Z 7. In order to write the C electron configuration we first need to know t. Therefore the C electron configuration will be 1s22s22p2. Since 1s can only hold two electrons the next 2 electrons for C goes in the 2s orbital. 2 See answers. On the removal of 2 valence electrons it will be become ...
4.6 Electronic configuration (ESABE) The energy of electrons (ESABF). The electrons of an atom all have the same charge and the same mass, but each electron has a different amount of energy.Electrons that have the lowest energy are found closest to the nucleus (where the attractive force of the positively charged nucleus is the greatest) and the electrons that have higher energy (and are able ...
Orbital Filling Diagram for Sulfur. what is the orbital diagram for sulfur the orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each the arrows solved show the orbital filling diagram for s sulfur st answer to show the orbital filling diagram for s sulfur stack the sub shells in order of energy with the ...
Double and triple bonds. What is the electron configuration and orbital diagram of. Box spin diagram of outer electron orbitals for the electron configuration of the 15 Phosphorus P 1s22s22p63s23p3 Ne3s 3p P pblock Gp5 Build the orbital diagram for the ion most likely formed by phosphorus. The rules for orbital filling diagrams. Energy 0 1 1 x 5.
Draw an orbital-filling diagram for the element with Z = 25, showing the individual electrons in the outermost subshell as up arrows, down arrows, or 0. How many partially filled orbitals does this...


























0 Response to "38 show the orbital filling diagram for n nitrogen"
Post a Comment