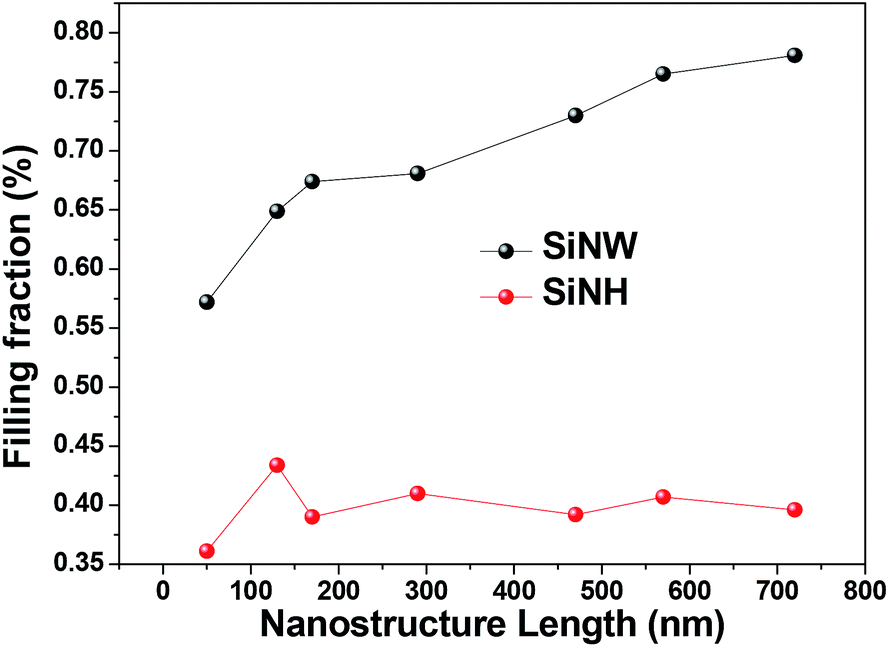
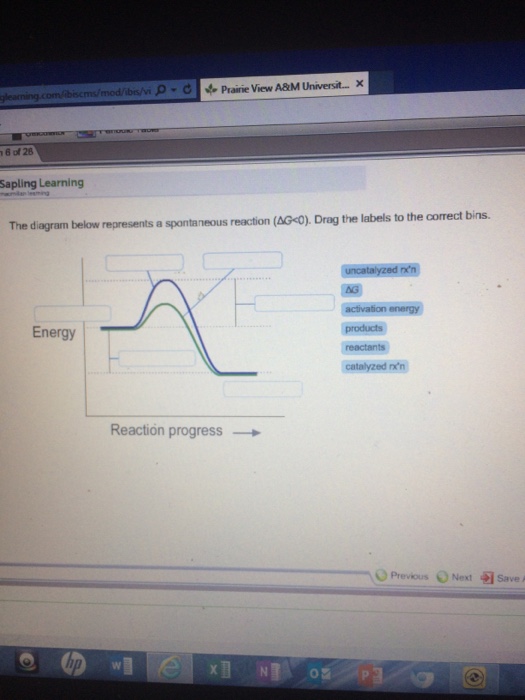
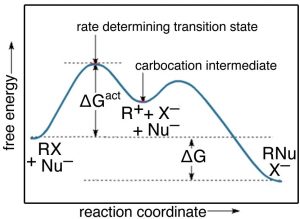
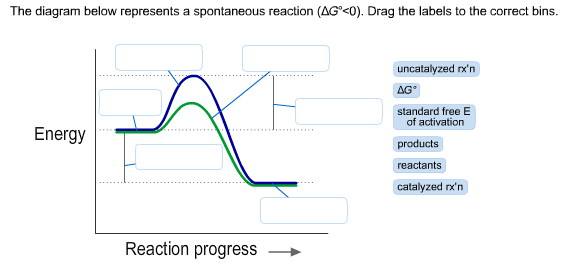
38 the diagram below represents a spontaneous reaction (δg°
Use Equation \(\ref{Eq5}\), the calculated value of ΔS°, and other data given to calculate ΔG° for the reaction. Use the value of ΔG° to determine whether the reaction is spontaneous as written. Solution. A To calculate ΔG° for the reaction, we need to know ΔH°, ΔS°, and T. We are given ΔH°, and we know that T = 298.15 K. The reaction is associated with a decrease in the number of particles in the same phase (3 mol→2 mol), so overall, a decrease in entropy: ΔS ⦵ for the reaction is negative. Hence, applying ΔG ⦵ = ΔH ⦵ ⦵, ΔG ⦵ will be negative (ΔG ⦵ <0), and the reaction spontaneous, if ΔH ⦵ >TΔS ⦵.
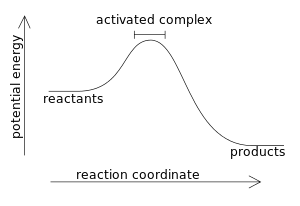
Question: Label the components of an energy diagram for a spontaneous reaction. Answer Bank reactants activation energy products uncatalyzed reaction catalyzed reaction Reaction progress- This problem has been solved! See the answer See the answer See the answer done loading.

The diagram below represents a spontaneous reaction (δg°
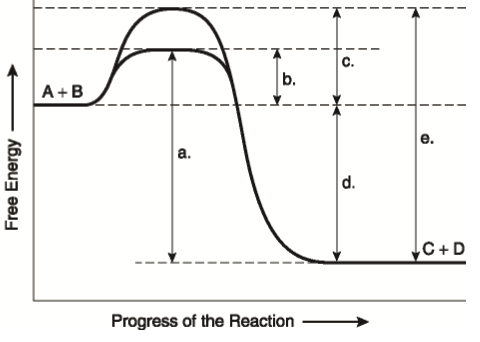
26.Given the potential energy diagram of a chemical reaction: Which arrow represents the potential energy of the reactants? 27. Base your answer to the following question on the potential energy diagram below, which represents the reaction: A + B ® C + energy. A) 1 B) 2 C) 3 D) 4 Which numbered interval will change with the addition A spontaneous reaction may involve an increase or decrease in enthalpy, it may involve an increase or decrease in entropy, but it will always involve a decrease in free energy that is a negative ΔG. A.The particle diagrams best represent that ΔH°<0 because the ions from both compounds are solvated by water molecules. B. The particle diagrams best represent that ΔH°<0 because both compounds produce about the same amount of CO 3 2− ions from the dissolution. C. The particle diagrams best represent that ΔS°>0 because both
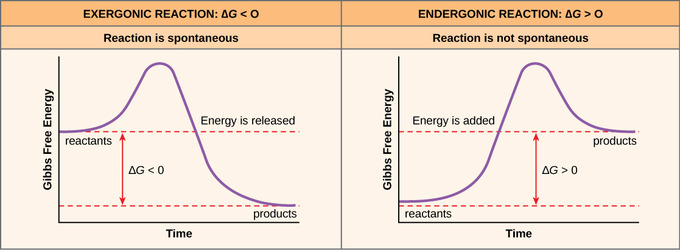
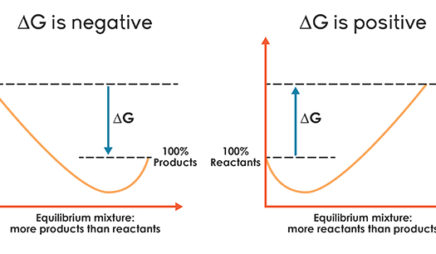
The diagram below represents a spontaneous reaction (δg°. Exergonic reactions are also called spontaneous reactions, because they can occur without the addition of energy. Reactions with a positive ∆ G (∆ G > 0), on the other hand, require an input of energy and are called endergonic reactions. In this case, the products, or final state, have more free energy than the reactants, or initial state. Electrical Work From Spontaneous Oxidation-Reduction Reactions. The following rule can be used to predict whether an oxidation-reduction reaction should occur.Oxidation-reduction reactions should occur when they convert the stronger of a pair of oxidizing agents and the stronger of a pair of reducing agents into a weaker oxidizing agent and a weaker reducing agent. chem1101 2014 j 12 june 2014 the diagram below represents this reaction involves an increase in the number of moles of gas so Δs will be positive as Δh = Δg tΔs this means that Δh Δg for the other reactions there is a decrease in the number of moles of has so Δs is negative Δh = Δg tΔs this means that Δh Δg The reaction SO2(g)+2H2S(g)⇌3S(s)+2H2O(g)SO2(g)+2H2S(g)⇌3S(s)+2H2O(g) is the basis of a suggested method for removal of SO2SO2 from power-plant stack gases. The values below may be helpful when answering questions about the process. Calculate the equilibrium constant KpKpK_p for the reaction at a temperature of 298 KK.
The diagram below represents a spontaneous reaction (δg (a) The reaction glyceraldehyde 3-phosphate 1,3-bisphosphoglycerate should be inhibited when levels of NADH fall. (b) The ΔG° for the oxidation of the aldehyde group on glyceraldehyde 3-phosphate to form a carboxylic acid is more negative than the ΔG° for ATP hydrolysis. Worked Example: E o for the Redox Reaction NOT Given. Question: A strip of magnesium metal is placed in an aqueous 1 mol L-1 copper(II) sulfate solution. Will a spontaneous redox reaction occur? Solution: (Based on the StoPGoPS approach to problem solving.). What is the question asking you to do? Determine if the redox reaction as given is spontaneous. For the reaction below ΔG° = + 33.0 kJ, ΔH° = + 92.2 kJ, and ΔS° = + 198.7 J/K. Estimate ... The figure below represents the spontaneous reaction of H2 (shaded spheres) with O2 (unshaded spheres) to produce gaseous H2O. ... According to the diagram above, ΔG° is positive and the equilibrium composition is rich in reactants. The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. a. Is the reaction endothermic or exothermic? b. What is the activation energy of the reaction?
14 Dec 2020 — The diagram below represents a spontaneous reaction. Drag the labels to the correct bins. Is the reaction endo the rmic or exo the rmic? The reaction will only be allowed if the total entropy change of the universe is zero or positive. This is reflected in a negative ΔG, and the reaction is called an exergonic process. ΔG = (-) -T(-): Higher values of T will cause the reaction to be less spontaneous, more positive. b. Some reactions that are predicted by their signs of ΔG° to be spontaneous at room temperature do not proceed at a measurable rate at room temperature. Account for this apparent contradiction. This is a separate question of reaction kinetics. The diagram below represents a spontaneous reaction δg institution. Chem1101 2014 j 12 june 2014 the diagram below represents this reaction involves an increase in the number of moles of gas so δs will be positive as δh δg tδs this means that δh δg for the other reactions there is a decrease in the number of moles of has so δs is ... 54.The spontaneous decay of an atom is called 55.The diagram below represents a nuclear reaction in which a neutron bombards a heavy nucleus. Afission Bfusion Calpha decay Dbeta decay Which type of reaction does the diagram illustrate? Acombustion Breduction Cnuclear fission Dnuclear fusion 56.In which type of reaction do two lighter nuclei combine
Energy reaction progress which curve represents the catalyzed reaction. Energy diagrams depict the reaction progress versus energy. First as noted the y axis is labeled enthalpy and the x axis is labeled reaction progress then we have the actual energy diagram plot. Identify the key features of the reaction profile below.
These are included in an equation to tell you whether a reaction happens: ΔG = ΔH - TΔS Depending on the combination of signs you have for these two state functions, you'll be able to determine whether a reaction is spontaneous or non-spontaneous, or whether that depends on temperature. Case #1: The reaction is always spontaneous.
Entropy is a mathematically defined property in thermodynamics. It can often help to understand it as a measure of the possible arrangements of the atoms, ions, or molecules in a substance. The symbol for entropy is S, and a change in entropy is shown as "delta" S or ΔS. If the entropy of a system increases, ΔS is positive.
ΔG > 0; the reaction is non-spontaneous and endergonic. ΔG < 0; the reaction is spontaneous and exergonic. ΔG = 0; reaction is at equilibrium. Note: According to the second law of thermodynamics entropy of the universe always increases for a spontaneous process. ΔG determines the direction and extent of chemical change.
ΔG represents the change in Gibbs free energy for a chemical system at constant temperature and pressure ΔG = ΔH -TΔS ... We can write an equation to represent this entropy change in the surroundings at constant temperature as shown below: ... For a spontaneous reaction, ΔG for the reaction is negative (ΔG < 0).
The diagram below represents a spontaneous reaction (δg. The spontaneous redox reaction in a voltaic cell has _____ A) a negative value of Ecell and a negative value of ΔG. B) a positive value of Ecell and a positive value of ΔG. C) a negative value of Ecell and a positive value of ΔG. D) a positive value of Ecell and a negative value of ΔG.
The diagram below represents a spontaneous reaction (deltaG degree < 0). Drag the labels to the correct bins. uncatalyzed rx'n Delta G degree standard free E of activation products reactants catalyzed rx?n. Question: The diagram below represents a spontaneous reaction (deltaG degree < 0).
Although we can write any chemical reaction on paper not all chemical reactions will occur. A spontaneous reaction is a chemical reaction that will occur because of the nature of the system once it is initiated. Show transcribed image text the diagram represents a reaction. Find an answer to your question use the diagram below for questions 28 ...
Use the following potential energy diagram to answer the questions below. The diagram represents a spontaneous reaction use the diagram to answer the questions below. Kj b determine the activation energy for the reverse reaction. Note that just because a reaction is called spontaneous does not necessarily mean that the reaction will happen ...
The diagram below represents a spontaneous reaction (ΔG°<0). Fill in tthe blanks below. Learn this topic by watching Gibbs Free Energy Concept Videos. All Chemistry Practice Problems Gibbs Free Energy Practice Problems. Q. The reaction SO2 (g) + 2H2S (g) ⇌ 3S (s) + 2H2O (g) is the basis of a suggested method for removal of SO2 from power ...
j. Based on your answers from c, g and h, determine if the following reaction is spontaneous. Explain. 2Ag(s) + Cu2+(aq) → Cu(s) + 2Ag+(aq) No, the reaction a written above is not spontaneous. Based on the spontaneous reaction written in part c, Cu(s) is more likely to give up electrons and Ag+(aq) is more likely to accept electrons.
A.The particle diagrams best represent that ΔH°<0 because the ions from both compounds are solvated by water molecules. B. The particle diagrams best represent that ΔH°<0 because both compounds produce about the same amount of CO 3 2− ions from the dissolution. C. The particle diagrams best represent that ΔS°>0 because both
A spontaneous reaction may involve an increase or decrease in enthalpy, it may involve an increase or decrease in entropy, but it will always involve a decrease in free energy that is a negative ΔG.
26.Given the potential energy diagram of a chemical reaction: Which arrow represents the potential energy of the reactants? 27. Base your answer to the following question on the potential energy diagram below, which represents the reaction: A + B ® C + energy. A) 1 B) 2 C) 3 D) 4 Which numbered interval will change with the addition


/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)







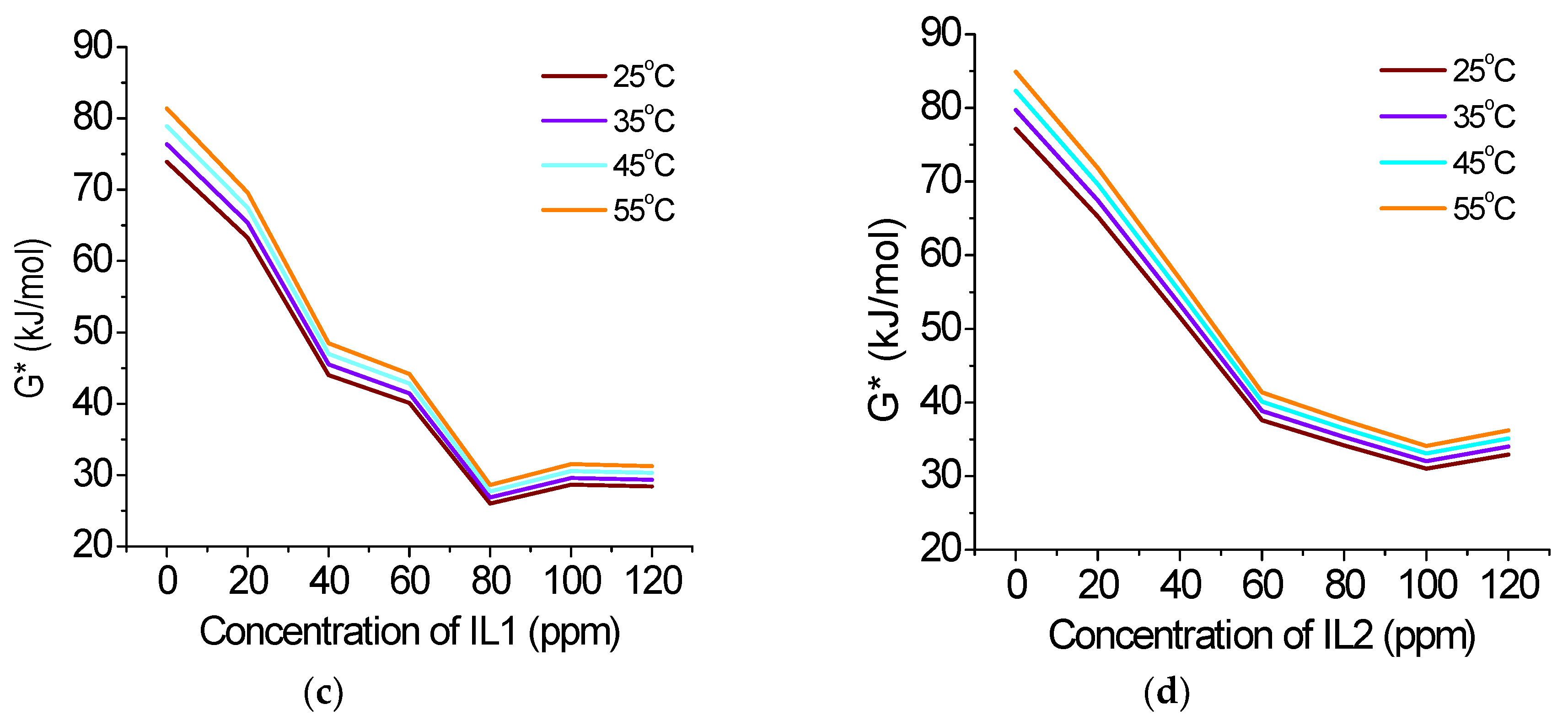



0 Response to "38 the diagram below represents a spontaneous reaction (δg°"
Post a Comment