41 energy diagram endothermic and exothermic reaction
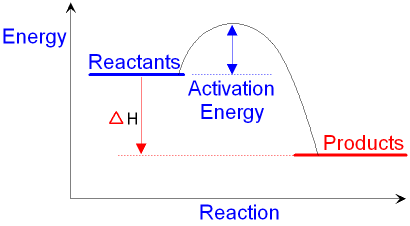
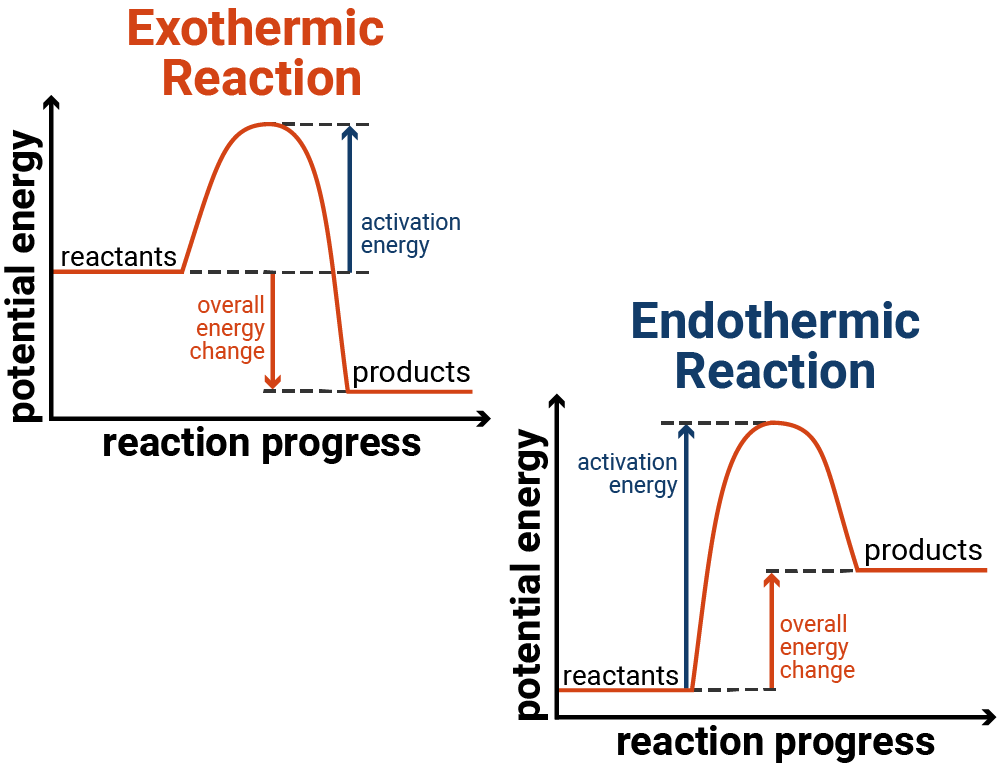
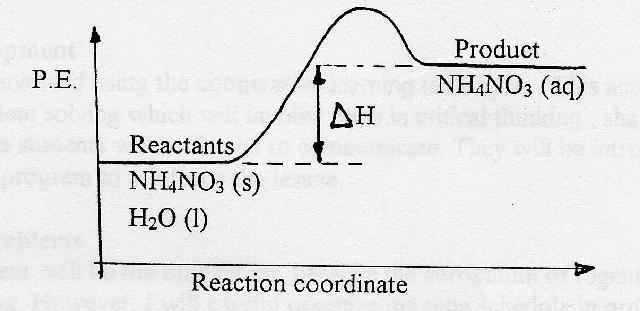
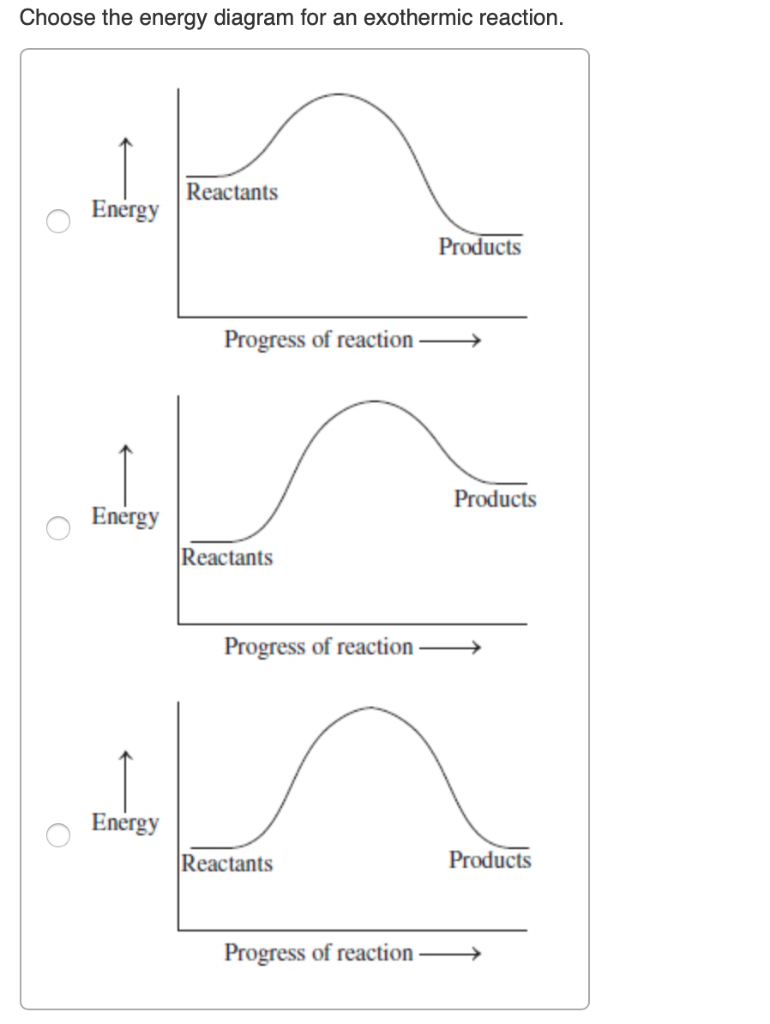
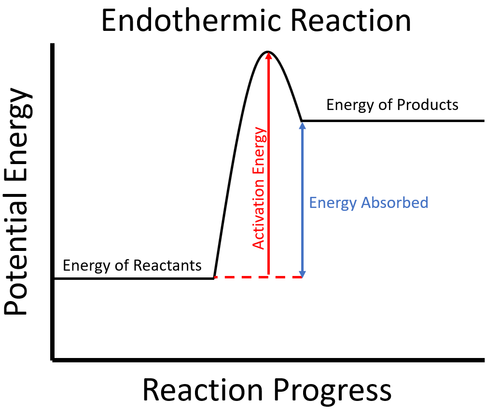
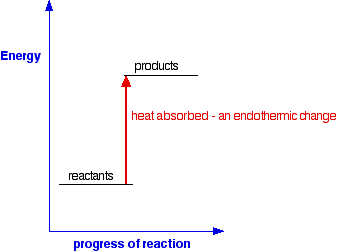
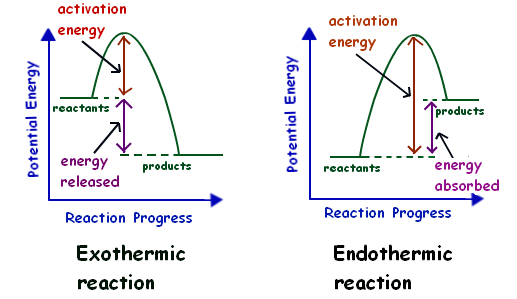
c) Draw a labeled enthalpy level diagram for an exothermic and endothermic reaction showing the activation energy, Ea and enthalpy change. [4] 9. (M05/S/2) In a neutralization reaction 50 cm 3 of a 0.50 moldm-3 solution of sodium hydroxide is mixed rapidly in a glass beaker with 50 cm 3 of a 0.050 moldm-3 solution of sulfuric acid. A reaction that takes in heat energy so the temperature goes down on a energy profile diagram is it a exothermic or endothermic reaction if the activation energy is small. In the case of an endothermic reaction the reactants are at a lower energy level compared to the productsas shown in the energy diagram below.
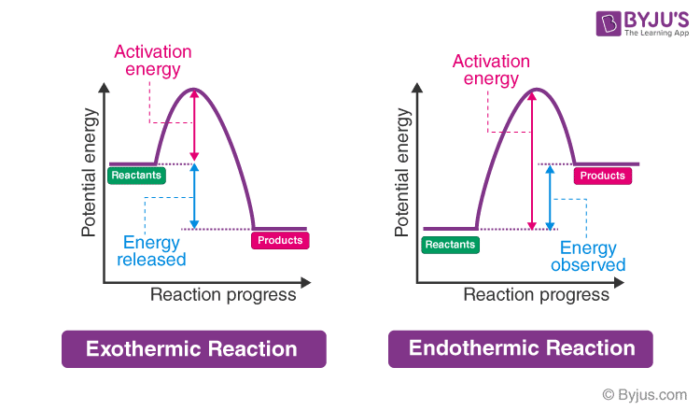
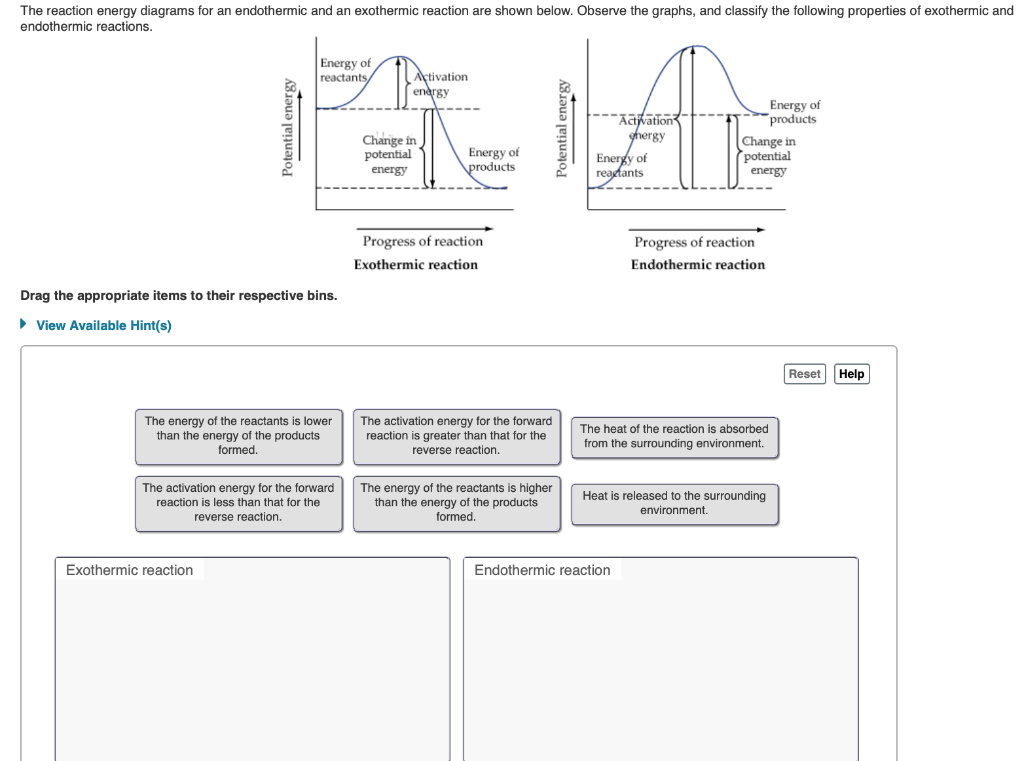
Transcribed image text: The reaction energy diagrams for an endothermic and an exothermic reaction are shown below. Observe the graphs, and classify the following properties of exothermic and endothermic reactions. Energy of reactants Activation energy Potential energy Potential energy Change in Energy of products Change in potential energy Activation ghergy Energy of reactants potential ...

Energy diagram endothermic and exothermic reaction
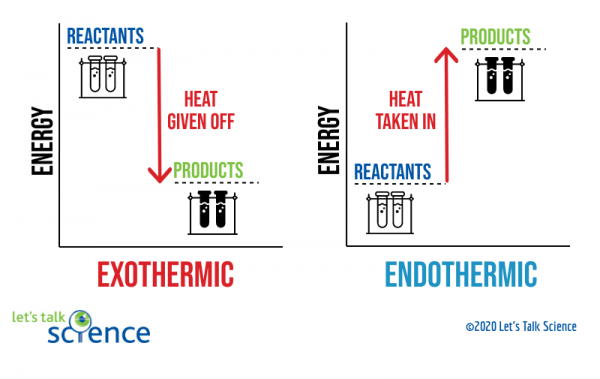
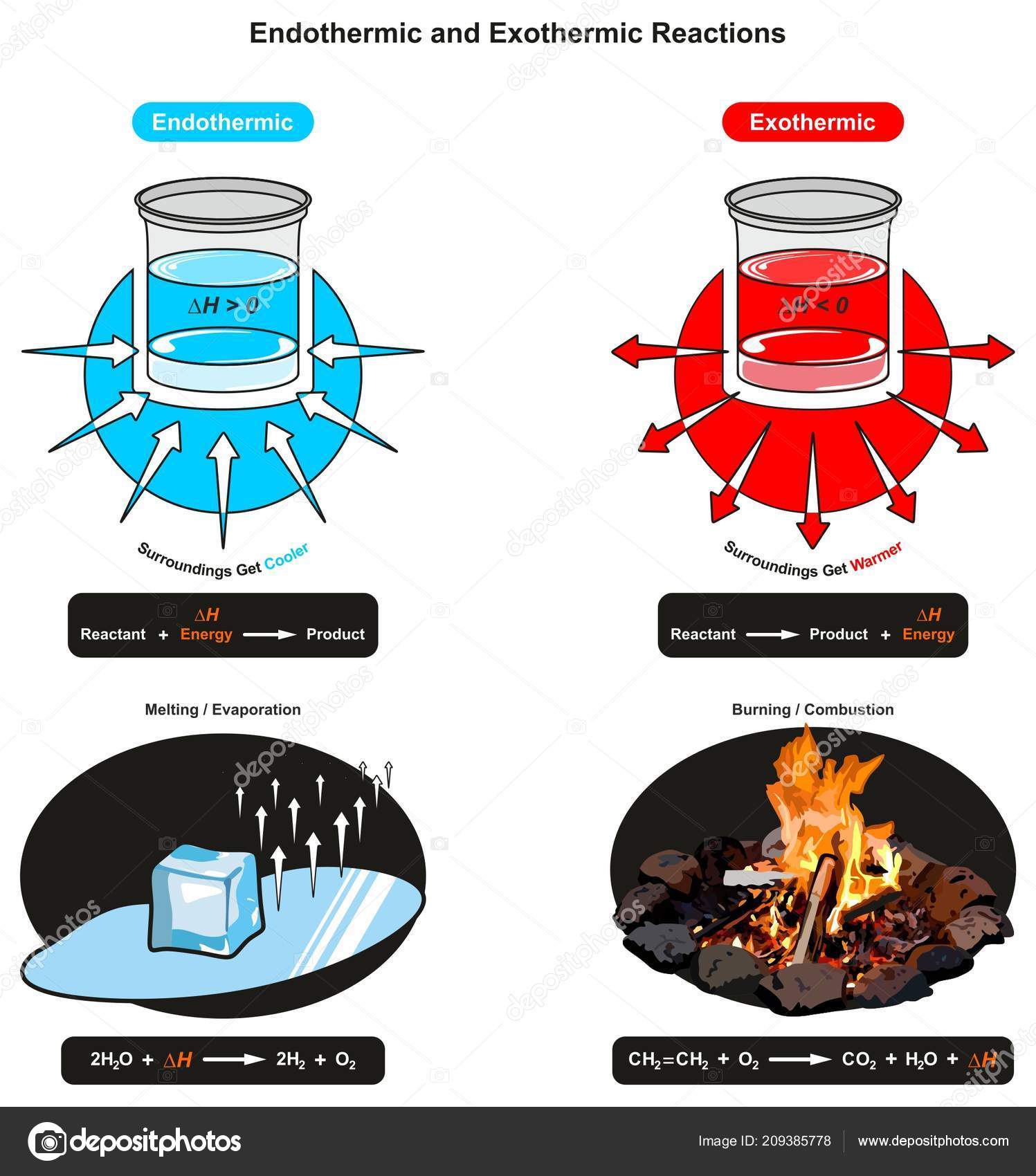
An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non-isolated system gains heat. Endothermic reactions result in an overall positive heat of reaction ( q r x n > 0 ). Exothermic and endothermic reactions cause energy level differences and therefore differences in enthalpy ( Δ H ... An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction Energy Diagrams. Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure \(\PageIndex{2}\). In endothermic reactions, the reactants have higher bond energy (stronger bonds) than the products. Strong bonds have lower potential energy than weak bonds.
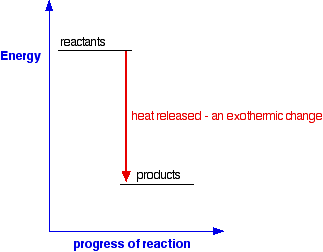
Energy diagram endothermic and exothermic reaction. Exothermic and Endothermic Reactions - Energy Level DiagramForm 5 Chemistry Chapter 4 ThermochemistryThis video is created by http://www.onlinetuition.com.my... Endothermic Diagram. Energy absorbed in reaction. Activation . Energy. Energy used in bond. breaking. Endothermic – more energy is taken in to break the bonds in the reactants than released by the bonds being formed in the products. Therefore, energy is absorbed. Energy released in bond making The exothermic reaction is the opposite of an endothermic reaction. It releases energy by light or heat to its surrounding. A few examples are neutralization, burning a substance, reactions of fuels, deposition of dry ice, respiration, solution of sulfuric acid into water and much more. Dec 01, 2020 · Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show: (a) the total energy content of the reactants compared to the total energy content of the products. Figure shows the energy level diagram of an exothermic reaction. Figure shows the energy level diagram of an endothermic reaction.
Representing endothermic and exothermic processes using energy diagrams. A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state. Endothermic reactions: Heat is absorbed. 1) Photosynthesis: Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. 6CO2 + 6 H2O + heat ---> C6H12O6 + 6O2. 2) Cooking an egg: Heat energy is absorbed from the pan to cook the egg. Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy. Energy Diagrams. Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure \(\PageIndex{2}\). In endothermic reactions, the reactants have higher bond energy (stronger bonds) than the products. Strong bonds have lower potential energy than weak bonds.
An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non-isolated system gains heat. Endothermic reactions result in an overall positive heat of reaction ( q r x n > 0 ). Exothermic and endothermic reactions cause energy level differences and therefore differences in enthalpy ( Δ H ...
Gcse Chemistry What Are Energy Level Diagrams What Is The Energy Level Diagram For An Exothermic Reaction Gcse Science
The Course Of A Reaction Figure 13 4 Plots The Course Of A Reaction The Initial Average Energy Of The Reactants Is Indicated At The Left Side Of Each Graph If Molecules Are To Collide Effectively They Must Have More Than The Average Energy They Must Have

A An Endothermic Reaction I Taking Place In A Test Tube What Would You Expect To Feel When You Brainly Com
What Is Difference Between Endothermic And Exothermic Reaction If Both Require Activation Energy Quora

Exothermic Energy Diagram Activation Energy Transition States And Enthalpy Change Tutor Hotline Youtube
What Is The Difference Between An Exothermic Reaction And Endothermic In Which Case We Use The Catalyst

Question Video Identifying Steps In A Reaction Profile Diagram For A Two Step Chemical Reaction Nagwa

Draw An Energy Diagram For An Exothermic Reaction Label The Activation Enthalpy And The Change In Enthalpy Delta H On The Diagram Study Com














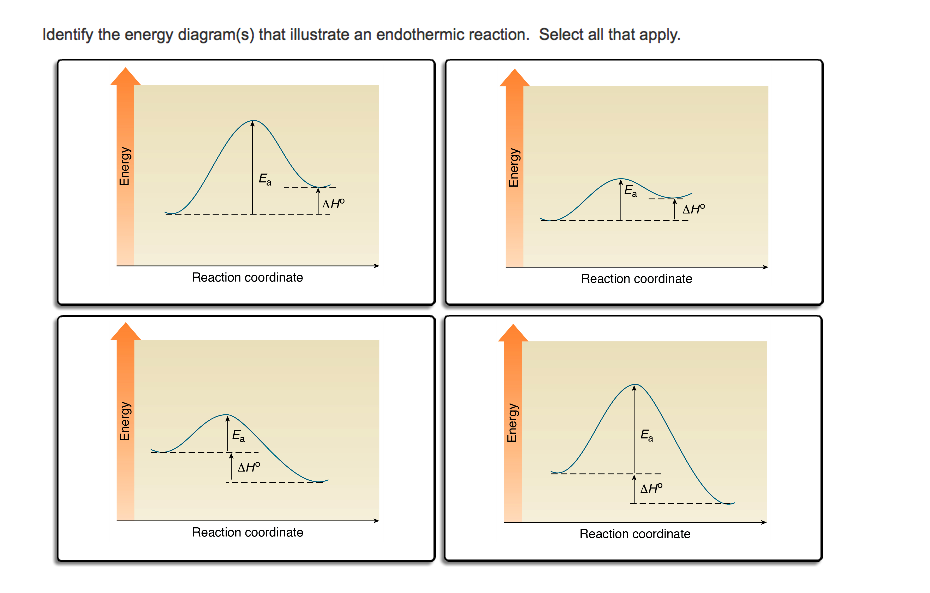
0 Response to "41 energy diagram endothermic and exothermic reaction"
Post a Comment