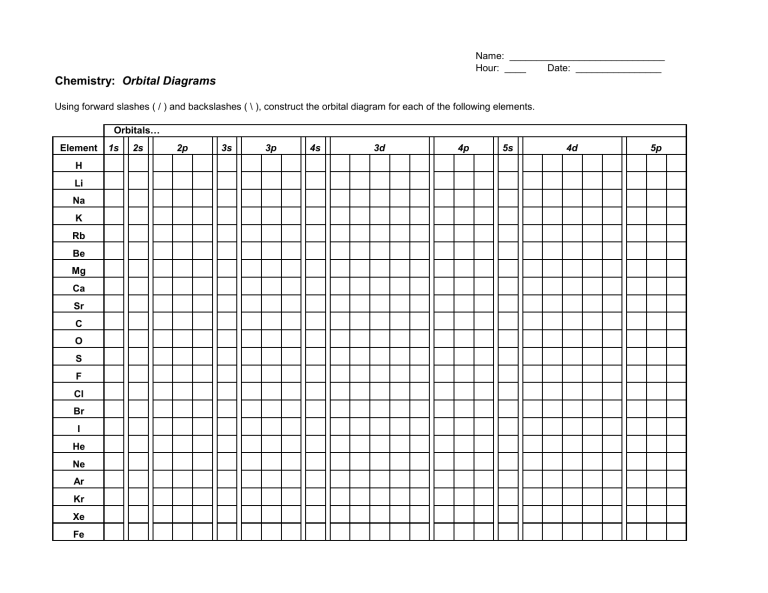
41 construct the orbital diagram for as
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau... Construct the molecular orbital diagram for dichlorine. x y z z y 3 x y z z y 4 Showing the p orbitals. Showing the s and p orbitals. ORBITALS AND MOLECULAR REPRESENTATION 11. CARBON ORBITALS Methane Ethane METHANE AND ETHANE C H H H H CH4 C C H H H H H H C2H6 1 2 Color conventions: Hydrogen atoms are shown in gray.
Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally.

Construct the orbital diagram for as
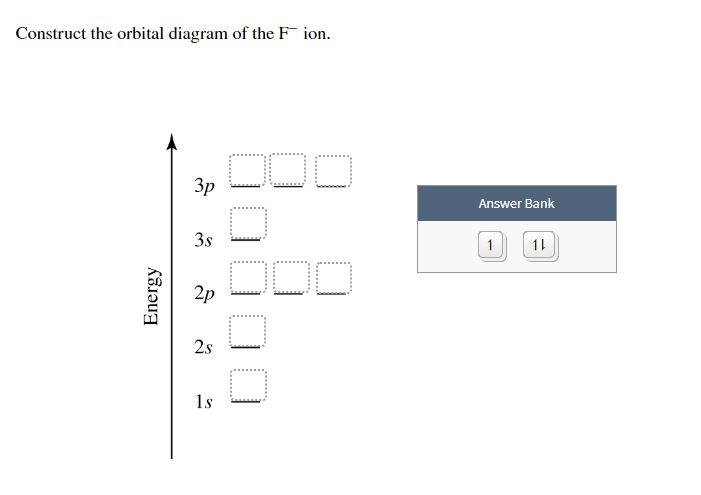
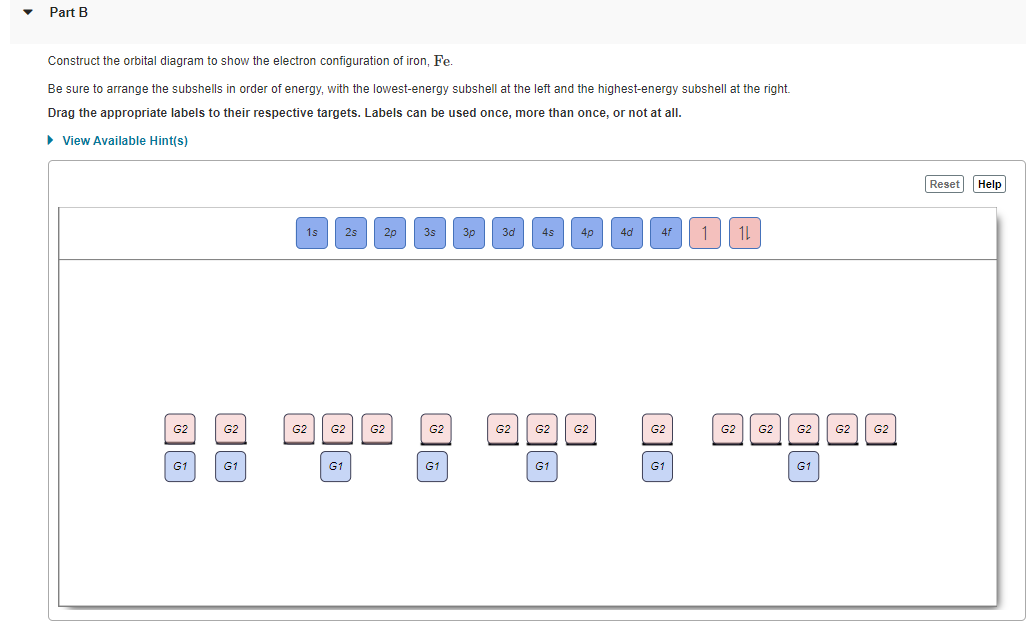
Part D Construct the orbital diagram for the ion . Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be filled. ANSWER: Help Reset G1 G1 G1 G1 G1 G1 G1 G1 G1 G1 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 1 s 4 d 2 s 4 f 2 p 5 ... The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org! Arsenic atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Construct the orbital Diagram Of the F- Ion. construct construct the orbital diagram the f- ion chem 120a november 8 2005 fall 2004 8 00 - 9 20 am exam ii name prepare a molecular orbital energy level diagram for no construct a solved construct the orbital diagram the f ion a ne answer to construct the orbital diagram of the f ion a neutral fluorine atom has 9 electrons how many ...
Construct the orbital diagram for as. FREE Expert Solution. We're being asked to construct the orbital diagram for N3-. For this problem, we need to do the following: Step 1: Determine the electron configuration of the neutral element. Step 2: Determine the electron configuration of the ion. Step 3: Construct the orbital diagram for the ion. 82% (439 ratings) Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid Orbitals 1. Begin with the Lewis structure. 2. Decide how many orbitals each atom needs to make its sigma bonds and to hold its non-bonding electrons. Draw the atomic and hybrid orbitals on on side of the page. 3. For each sigma bond, take a hybrid (or atomic) orbital from ... Construct The Orbital Diagram For As Hints Clutch Prep. Solved Identify The Orbital Diagram Of Ti T2 And Tr Ti Chegg Com. Draw And Explain The Orbital Diagram For Titanium Study Com. Construct The Orbital Diagram Of Each Atom Clutch Prep. Share this post. 0 Response to "40 orbital diagram for ti" Answer to: Draw and explain the orbital diagram for copper (Z = 29). By signing up, you'll get thousands of step-by-step solutions to your homework...
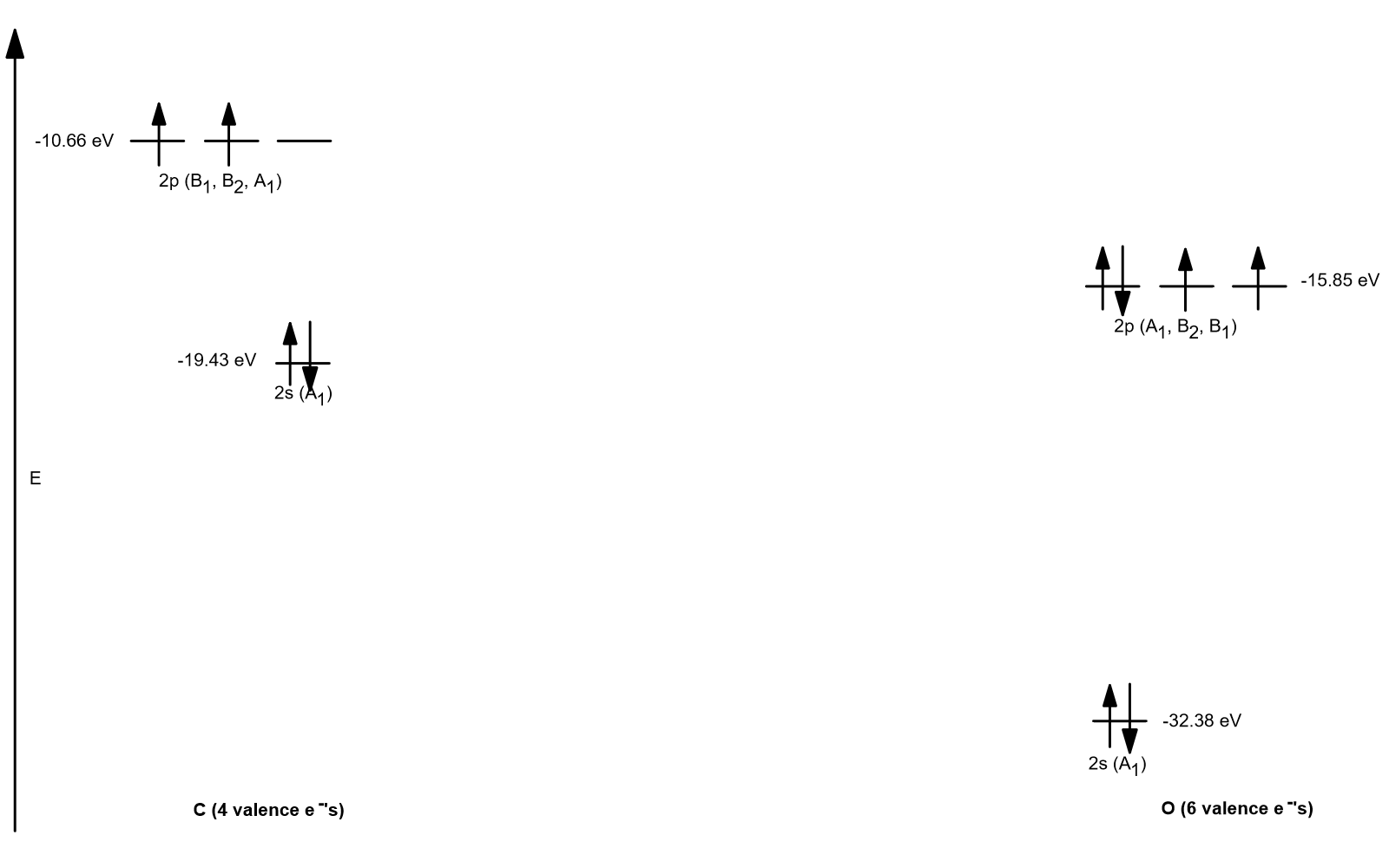
Draw the atomic orbital diagrams for the two exceptions and indicate how many unpaired electrons are present. Atomic Orbital Diagram: Electrons are situated in the various orbitals in an atom. Orbital Interaction Diagram 1. Plot atomic valence orbital en ergies (or fragment orbitals for More complex molecules). 2. Determine which orbitals can interact (those with S 0). 3. Determine magnitude of each interaction: scales directly with magnitude of overlap scales inversely with orbital energy difference 4. Plot MO energies and draw orbitals Answer to Construct the orbital diagram for Ni. Start by adding the appropriate subshells. For example, carbon is in the 2p block.1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
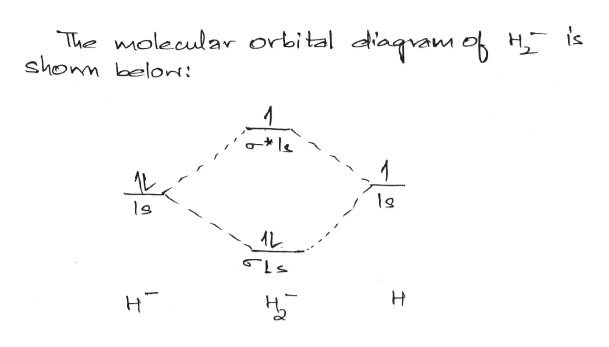
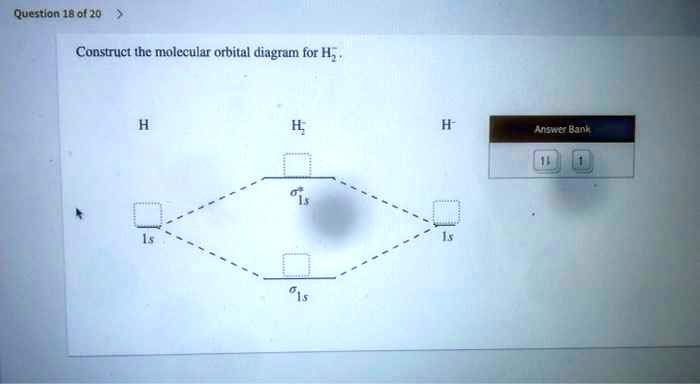
To draw the orbital diagram, begin with the following observations: the first two electrons will pair up in the 1s orbital; the next two electrons will pair up in the 2s orbital. According to Hund's rule, all orbitals will be singly occupied before any is doubly occupied. Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add %(8). Question: Orbital Diagrams Draw an orbital diagram for boron. Use this tool to draw the orbital diagram. Transcribed image text: Draw the molecular orbital diagram for Hez Drag the appropriate labels to their respective targets. Reset th Atomic orbital Molecular orbitals Atomic orbital 11 ॥ Antibonding ls ls Energy # He atom Bonding Het ion 1+1 11 He2+ ion. Previous question. Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Construct the orbital diagram of each atom or ion. In writing the electron configurat ion for fluorine the first two electrons will go in the 1s orbital. The 24 electrons of a. Ion electron confugurat ion s. A neutral fluorine atom has 9 electrons. Show transcribed image text construct the orbital diagram of the f ion. Draw the orbital diagram ...
Draw the orbital diagram for the valence shell of. Use an orbital diagram to describe the electron co. The orbital filling diagram of boron. After the 1s orbital is a 2s 2 electrons are going to go in there as well. Okay lets do the orbital diagram for iron iron we know is on its ground state of 26 electrons so we know the first electrons are ...
An orbital diagram is similar What is the orbital diagram for. For example, write the electron configuration of scandium, Sc: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. So for scandium the 1 st and 2 nd electron must be in 1s orbital, the 3 rd and 4 th in the 2s, the 5 th through 10 th in the 2p orbitals, etc. 6/14/ Ch 8 4/18 Correct Part B Complete ...
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 90% (29 ratings) Transcribed image text: Construct the orbital diagram for arsenic. Answer Bank Energy.
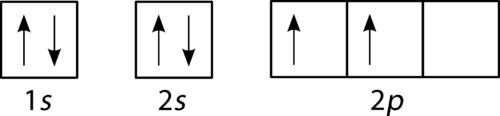
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
Construct the orbital diagram for As. First, we need to determine the electron configuration for As (arsenic). The electron configuration depends on the number of electrons an atom or ion has. Since As is neutral (uncharged), we can say that Z (atomic number) = number of protons = number of electrons. Arsenic has an atomic number of 33, so it ...
Construct the orbital Diagram for as. construct construct the orbital diagram for as a b a b 2 be same symmetry i e transform as the same construct a "relative" molecular orbital energy diagram f interpret the construct the orbital diagram for as imageresizertool construct the orbital diagram for as thanks for visiting our site this is images about construct the orbital diagram for as posted ...
The rules for orbital filling diagrams. If you want to learn how to draw orbital filling diagrams, you need to follow these handy rules. They probably won't make sense right now, but I'll explain them when the time is right. For now, trust me that these rules are handy ones: Electron configurations list the orbitals from lower to higher ...
Construct the orbital diagram for as. Given the valence electron orbital level diagram a. Given the valence electron orbital level diagram a. Expert answer 100 14 ratings 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f 6s 6p 6d 7s 7p ok starting from 1s you have to go diagonally view the full answer.
Construct the orbital Diagram Of the F- Ion. construct construct the orbital diagram the f- ion chem 120a november 8 2005 fall 2004 8 00 - 9 20 am exam ii name prepare a molecular orbital energy level diagram for no construct a solved construct the orbital diagram the f ion a ne answer to construct the orbital diagram of the f ion a neutral fluorine atom has 9 electrons how many ...
The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org! Arsenic atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table.

1 Create The Atomic Orbital Of Diagram For Nitrogen 2 Construct The Orbital Diagram For Ni Homeworklib
Part D Construct the orbital diagram for the ion . Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be filled. ANSWER: Help Reset G1 G1 G1 G1 G1 G1 G1 G1 G1 G1 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 G2 1 s 4 d 2 s 4 f 2 p 5 ...

Orbital Diagrams Construct The Orbital Diagram Of Each Atom Or Ion What Is The Electron Configuration Homeworklib
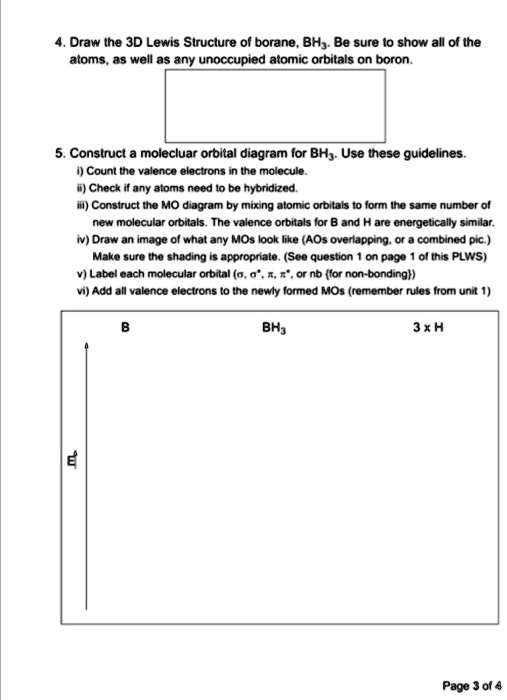
Solved Draw The 3d Lewis Struclure Of Borane Bh Be Sure Show All Of The Atoms As Well As Any Unoccupied Atomic Orbitals On Boron Construct Molecluar Orbital Diagram For Bhj Use Ihese

Atomic Orbital Electron Configuration Molecular Orbital Diagram Iron Ferric Iron Angle White Electronics Png Pngwing

Show The Full Ground State Electron Configuration Of Arsenic By Building Its Orbital Diagram What Are The Charges Of The Monatomic Ions Most Likely To Be Formed Select Two Charges An Anion
Solved Construct An Orbital Diagram To Show The Electron Configuration For A Neutral Magnesium Atom Mg Mg Drag The Appropriate Labels To Their Res Course Hero























0 Response to "41 construct the orbital diagram for as"
Post a Comment