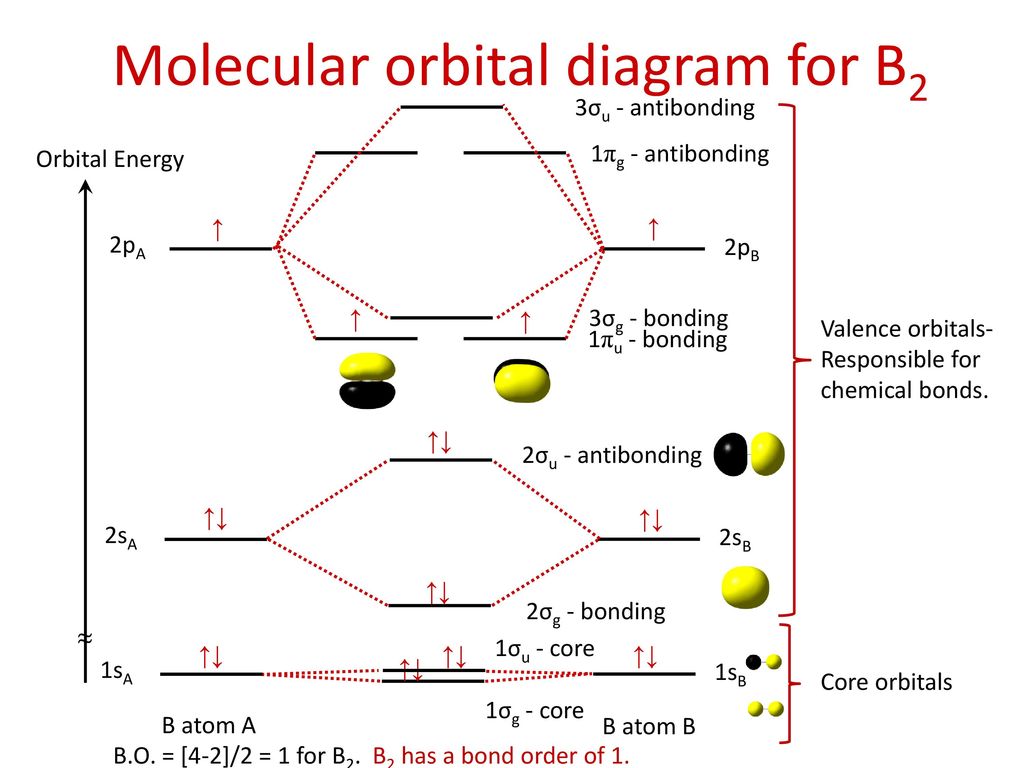
40 mo diagram of b2
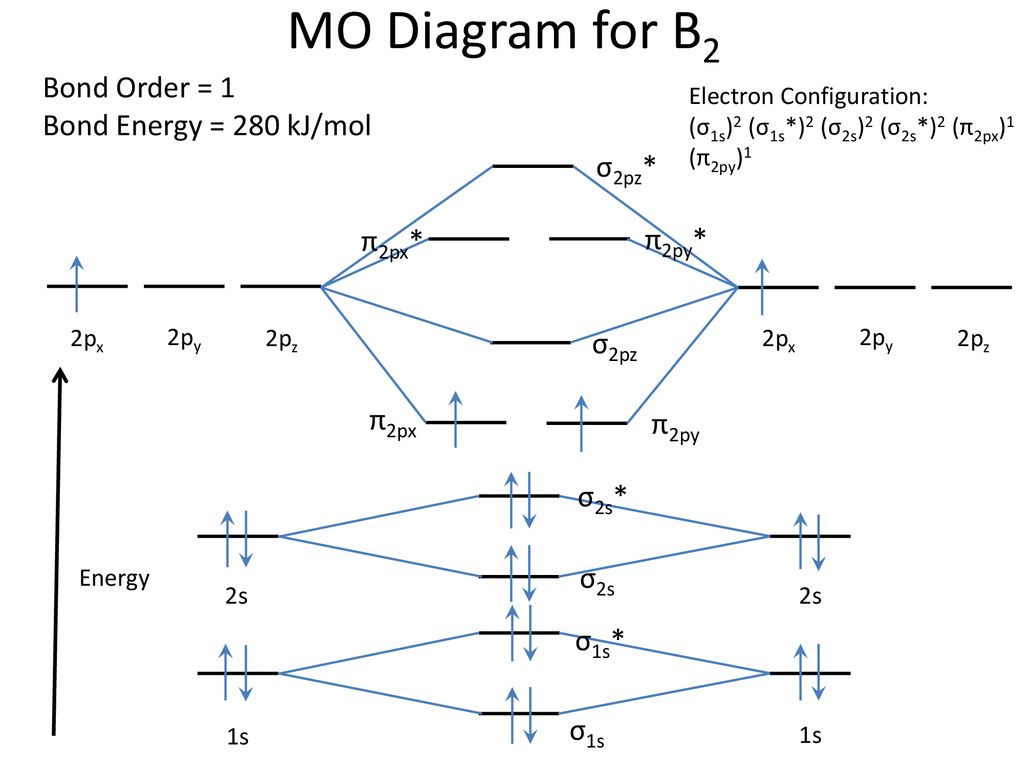
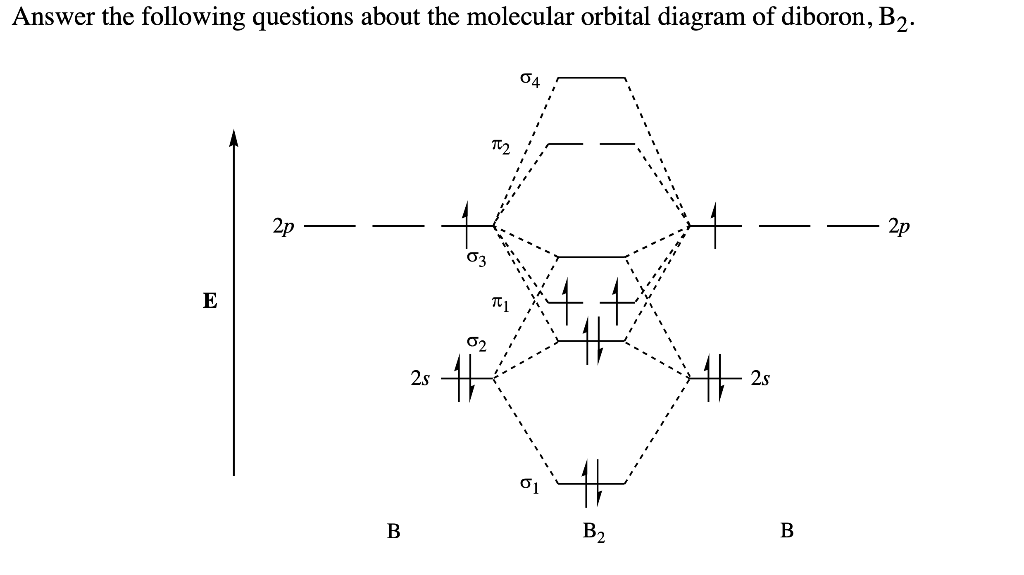
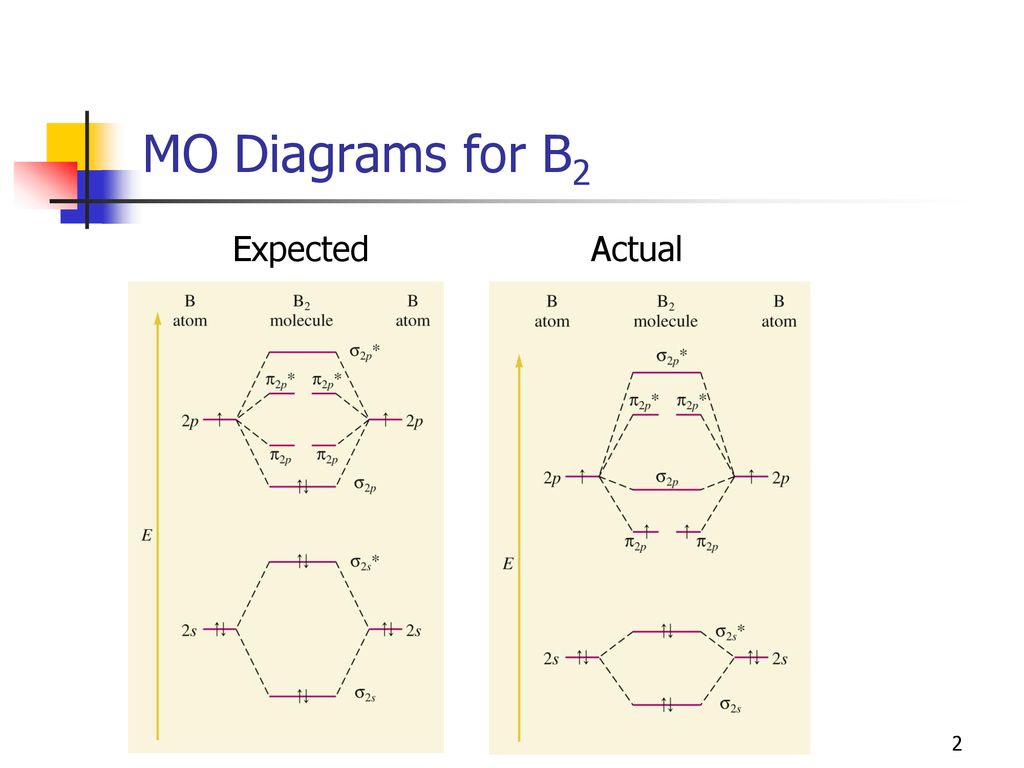
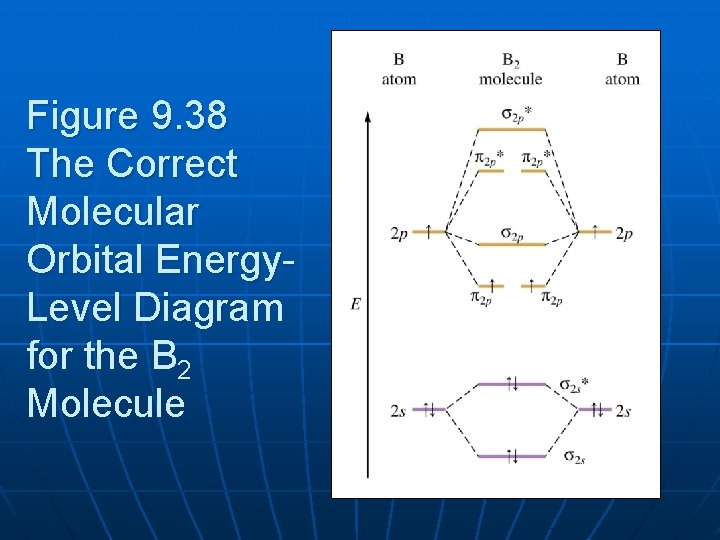
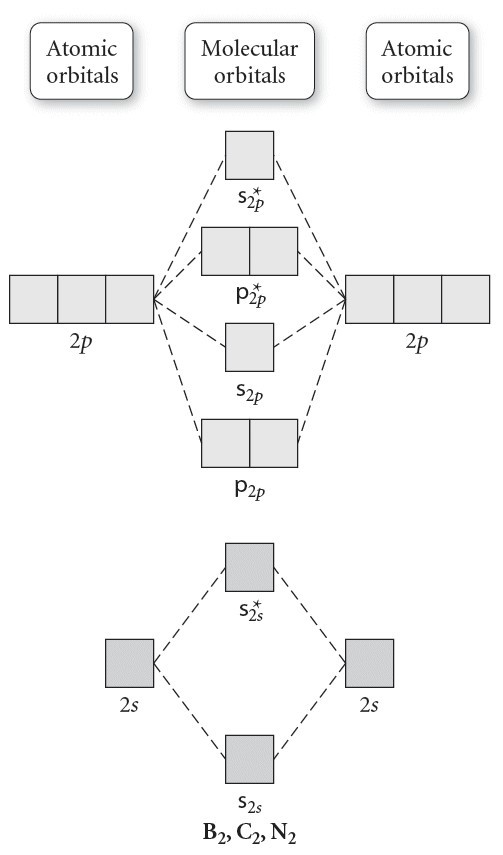
The resulting diagram looks (in approximation) like this: If you now fill the three valence electrons of each boron into the diagram according to Hund's rule, you will see that each π orbital will get one electron. This results in a triplet ground state. The finished valence molecular orbital diagram is pictured below. Neither. We’re being asked to determine the bond order of B2- and determine if it is paramagnetic or diamagnetic. For this, we need to determine the bond order for each species. Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion.
6:08This video discusses how to draw the molecular orbital (MO) diagram for the B2(-) molecule. The bond order ...2 Jun 2021 · Uploaded by Principia
Mo diagram of b2
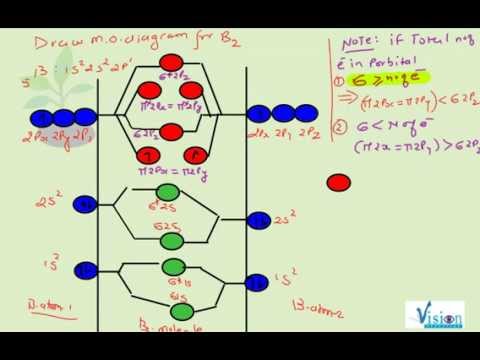
The molecular orbital diagram for B 2 molecule is as follows: We know that bond order is the difference between the number of bonds and the antibonds. Now, we have to calculate the bond order of B 2 molecule using the formula as follows: Bond order = 1 2 ( Number of electrons in BMO) − ( Number of electrons in ABMO) From the diagram, we can ... B2+ molecular orbital diagram. The electronic configuration of bez 4 is. The molecular orbital diagram for b₂ then becomes the video below describes how to generate molecular orbital diagrams for b₂ and other diatomic molecules from row 2 elements of the periodic table. We use the pauli exclusion principle and hunds rule to fill the ... B2 molecular orbital diagram. Since bond order is zero be 2 molecule does not exist. This was on a quiz and i somehow got the bond order and the lumo indicated wrong. I also calculated the bond order of this molecule to be 32. B 2 molecule is formed by the overlap of atomic orbitals of both boron atoms.
Mo diagram of b2. Use MO diagrams to place B2+, B2, and B2- in order of (a) decreasing bond energy; (b) decreasing bond length. Explanation. The electron configuration of . B B B. atom is . 1 s 2 2 s 2 2 p 1 1s^2 \\ 2s^2 \\ 2p^1 1 s 2 2 s 2 2 p 1. At first, build the molecular orbital diagram for . 6:08This video discusses how to draw the molecular orbital (MO) diagram for the B2 (boron) molecule. The bond ...30 May 2021 · Uploaded by Principia 14:24This video shows the end of the Be2 molecule MO diagram and explains pi orbitals, paramagnetism, and the ...26 Mar 2014 · Uploaded by Diego Troya 10:50From the periodic table as we have already discussed the Molecular orbital diagrams of diatomic molecules of ...24 Jun 2020 · Uploaded by Edmerls
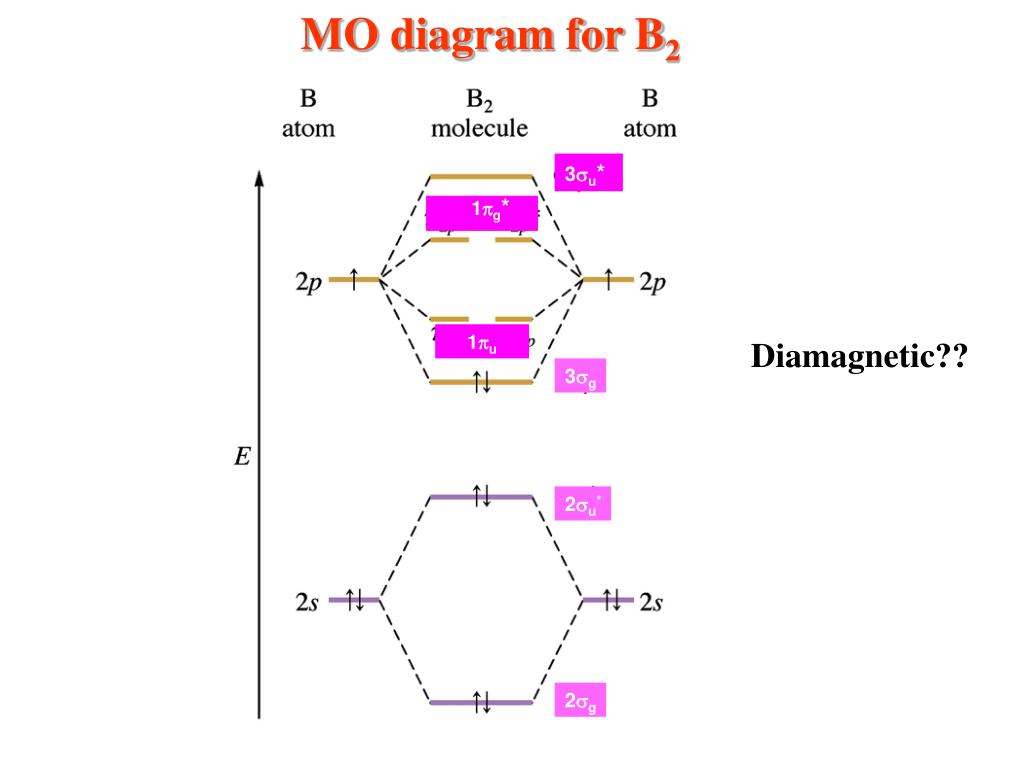
MO electronic configuration:Bond order: Here Nb = 4, Na = 2Bond order = The two boron atom is B2 molecules are linked by one covalent bond. Rating: 4,4 · 740 votes · Free · Android · Educational Re: M.O. Diagram for B2 Post by Chem_Mod » Tue Nov 11, 2014 11:21 pm As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!) The number of unpaired electrons in the B2+ molecule is 2 (two) 1 (one) 3 (three) zero ; Question: Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!) The number of unpaired ... B2 molecular orbital diagram. This also causes a large jump in energy in the 2p σ orbital. For example when two hydrogen atoms bond a σ1s bonding molecular orbital is formed as well as a σ1s antibonding molecular orbital. Valence bond model vs. The molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both ...
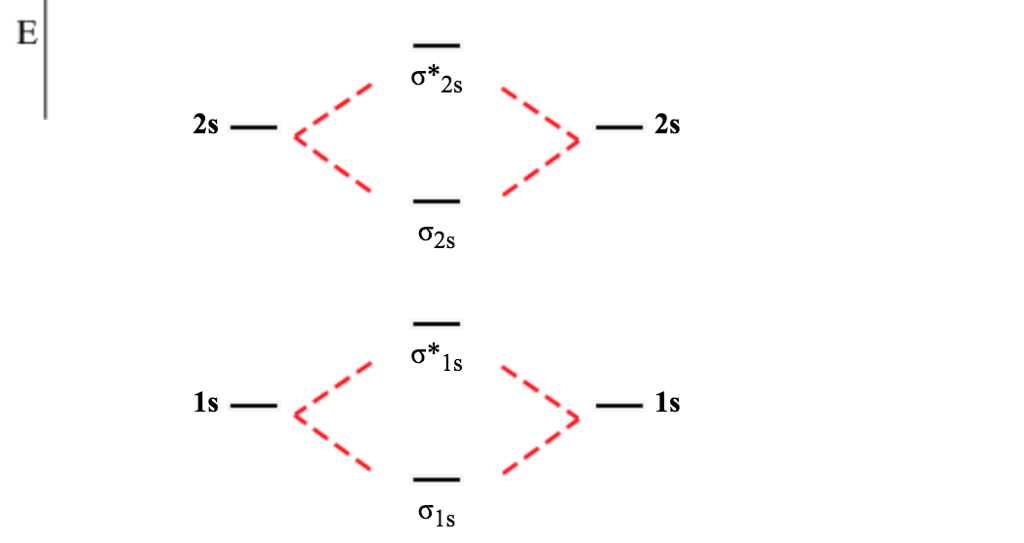
Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ... 6:25This video discusses how to draw the molecular orbital (MO) diagram for the B2(+) molecule. The bond order ...4 Jun 2021 · Uploaded by Principia B2 molecular orbital diagram. Since bond order is zero be 2 molecule does not exist. This was on a quiz and i somehow got the bond order and the lumo indicated wrong. I also calculated the bond order of this molecule to be 32. B 2 molecule is formed by the overlap of atomic orbitals of both boron atoms. B2+ molecular orbital diagram. The electronic configuration of bez 4 is. The molecular orbital diagram for b₂ then becomes the video below describes how to generate molecular orbital diagrams for b₂ and other diatomic molecules from row 2 elements of the periodic table. We use the pauli exclusion principle and hunds rule to fill the ...
The molecular orbital diagram for B 2 molecule is as follows: We know that bond order is the difference between the number of bonds and the antibonds. Now, we have to calculate the bond order of B 2 molecule using the formula as follows: Bond order = 1 2 ( Number of electrons in BMO) − ( Number of electrons in ABMO) From the diagram, we can ...

Draw The Molecular Orbital Diagram For I Be2 Ii B2 And Predict Bond Order And Magnetic Properties From Chemistry Chemical Bonding And Molecular Structure Class 11 Haryana Board English Medium

Scielo Brasil A Brief Introduction To Molecular Orbital Theory Of Simple Polyatomic Molecules For Undergraduate Chemistry Students A Brief Introduction To Molecular Orbital Theory Of Simple Polyatomic Molecules For Undergraduate

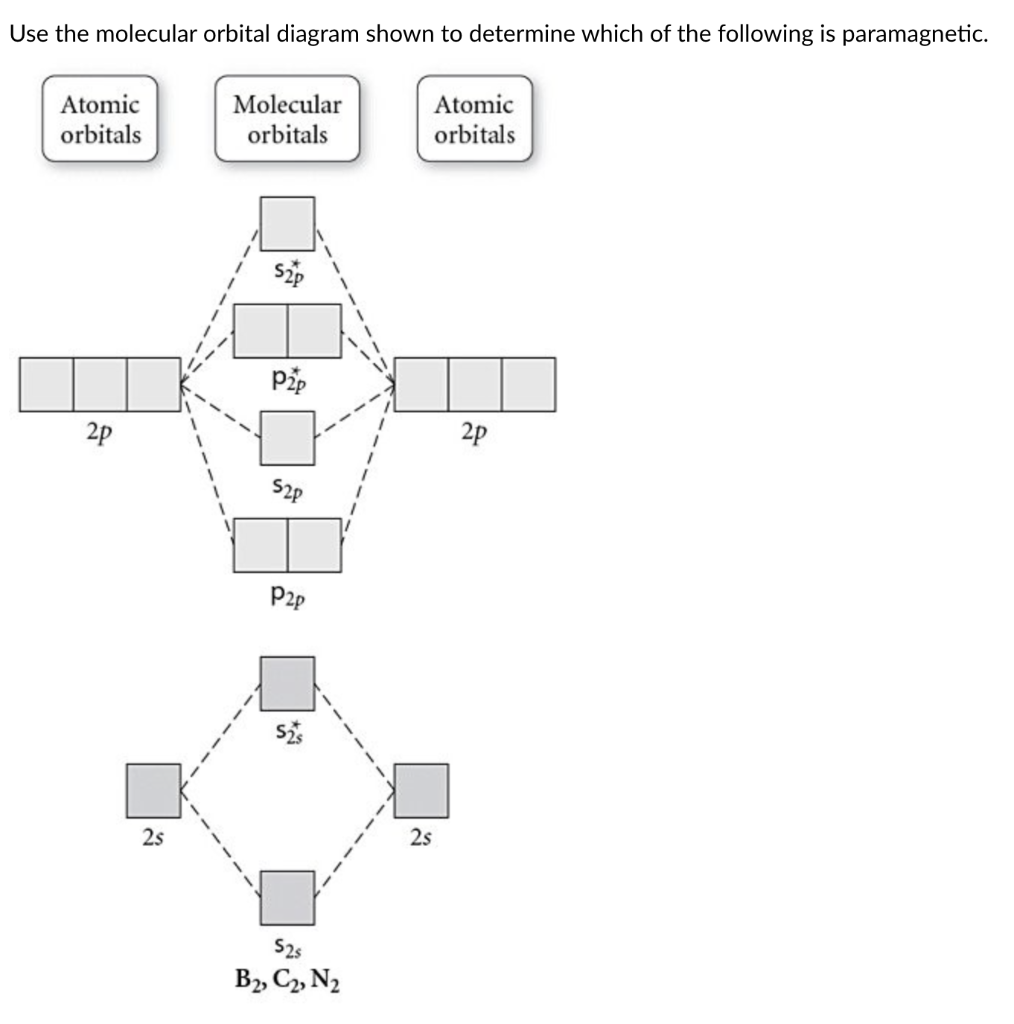
A Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic B 2 2 B2 C 2 2 B 2 2 And N 2 2 B Draw The Lewis Structures And Molecular Orbital Diagrams For














0 Response to "40 mo diagram of b2"
Post a Comment