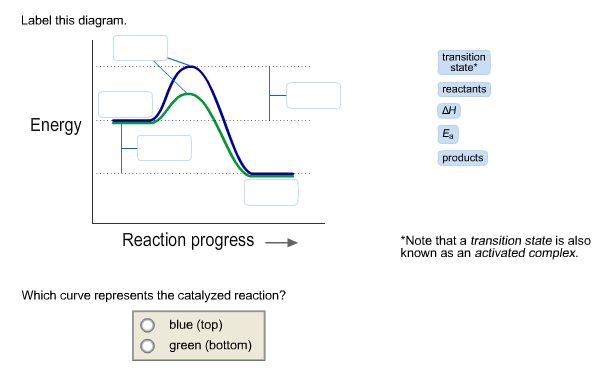
40 click on the point of the energy diagram that represents the activated complex (transition state).
The Energy Transition Index (ETI) is a fact-based ranking intended to enable policy-makers and businesses to plot the course for a successful energy transition. Key points of differentiation are on environmental sustainability, capital and investment in new energy infrastructure, and the inertia from... A nonequilibrium state that may persist for a very long time. phase diagram. A horizontal line constructed across a two phase region of a binary phase diagram; its intersections with the phase boundaries on either end represent the equilibrium compositions of the respective phases at the...
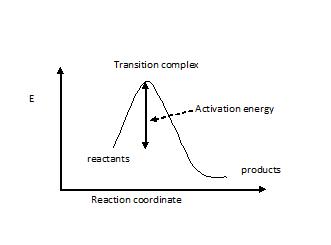
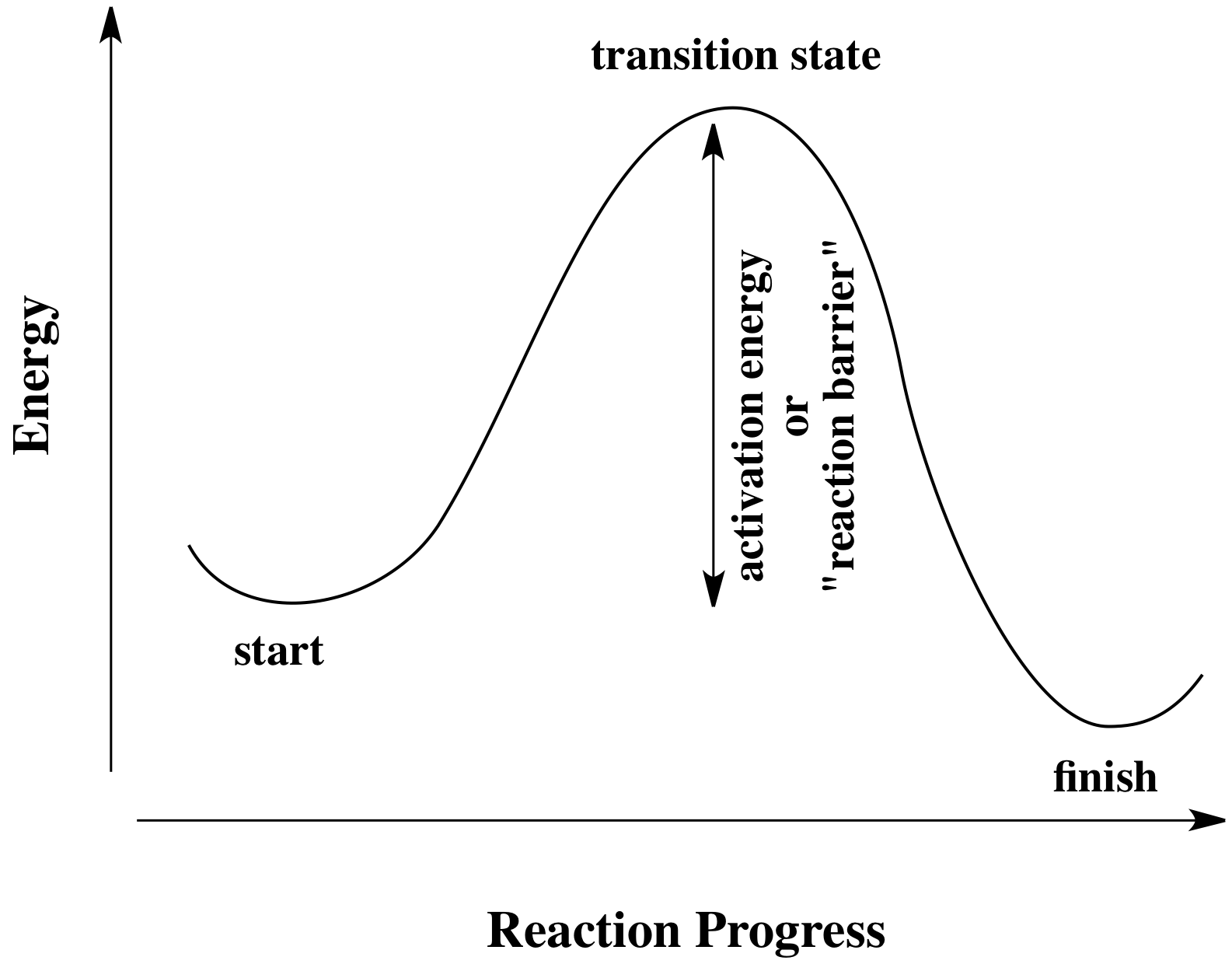
Transition states are local energy maximums and have partial bonds. This might be one of the reasons why they cant be isolated as intermediates. A transition state is a chemical species which has only fleeting existence and represents an energy maxima on reaction coordination diagram .
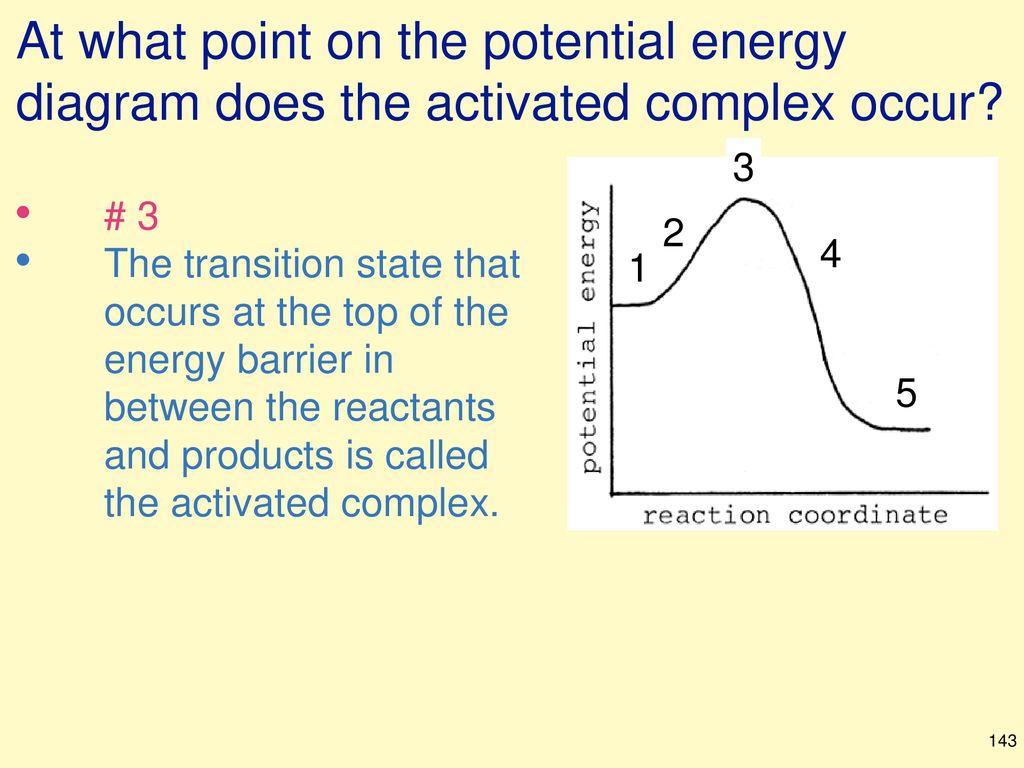
Click on the point of the energy diagram that represents the activated complex (transition state).
transition-state theory, also called activated-complex theory or theory of absolute reaction rates, treatment of chemical reactions and other processes that The difference between the energies of the transition and the initial states is closely related to the experimental activation energy for the... Click hereto get an answer to your question Energy of the activated complex is.activated complex is measured from the very bottom of the diagram to the top of the activation energy.On adding 0.095mol of Hg(CN)2 the freezing point of solution was −0.53∘C Assuming that complex is... Phase diagram is a graphical representation of the physical states of a substance under different A phase transition is the transition from one state of matter to another. There are three states of Imagine a substance with the following points on the phase diagram: a triple point at .5 atm and -5°...
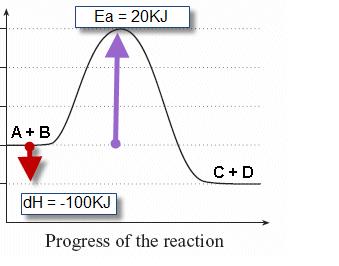
Click on the point of the energy diagram that represents the activated complex (transition state).. Then click on the active text for the quantity you wish to calculate. Since the change in Potential energy of an object between two positions is equal to the work that must be done to move the object from one point to the other, the calculation of potential energy is equivalent to calculating the work. 39 Activation Energy Activation Energy (Ea) minimum energy required for a reaction to occur Activated Complex: the transitional structure in a collision that exists while Transition State: the highest point on the energy diagram, representing the point at which the reaction is half-completed. That activation in turn goes into the next level as input and the second layer calculates weighted sum on that input and it in turn, fires based on another linear activation function. Sigmoid functions are one of the most widely used activation functions today. Then what are the problems with this? complex and reactants is termed as the activation energy (Ea). Figure 1. Potential energy diagram (potential energy profile or reaction coordinate where E0is an energetic difference between the energy of activated state (product) and reac-. tants that is given as relative to the energy of atoms in...
A transition to the enclosing state represents a transition to the initial pseudostate in each region. A newly-created object takes its topmost default transitions An entry point is shown as a small circle on the border of the state machine diagram or composite state, with the name associated with it. the vibrational frequency of the activated complex in the degree of freedom corresponding to its decomposition. It is therefore the frequency of The effect on the activation barrier for a reaction involving an H/D atom is shown below. Since the transition state is (usually) more loosely bound... The energy needs of 10 billion inhabitants of the Earth cannot be best addressed with the tools available today. Somehow the same situation could be true for electricity management in most of the contexts of the energy transition we are experiencing: the solution is information technology, but not... Answer to on the energy diagram that represents the activated complex transition state identify the point answer bank activated. The energy of the activated complex if the reaction is exothermic describe the positions of the reactants and products on a potential energy graph.
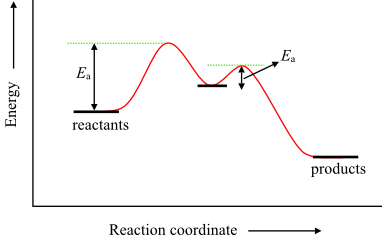
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes. The energy transition is already happening at a rapid pace, as businesses, utilities, consumers, and other stakeholders embrace the benefits The move to electric heat (powered by renewable sources) alone represents perhaps the single largest potential driver for expanding residential electrification. A theoretical linear relationship between activation energy and ionic strength was confirmed by application of the above data. The structure of the activated complex for NH 4 + oxidation to NO2 - was This represents the first known attempt to apply transition state concepts to the nitrification... Transition State Theory (Wigner, Eyring 1930s). Identify a 3N-1 dimensional dividing surface, a Transition State (TS), that represents a bottle neck for going from • Need to nd all relevant saddle points on the potential energy rim surrounding the energy basin corresponding to the initial state.
It states that the electronic transitions are so rapid and their timescale is so fast as compared with the nuclear motion that we may consider the nuclei to be fixed during the transition. Also, an electronic transition is most likely to occur when the nuclei are in their extreme positions on the potential...
Difference Between Activated Complex And Transition State Compare The Difference Between Similar Terms
The line at energy E represents the constant mechanical energy of the object, whereas the kinetic and potential energies Quartic and Quadratic Potential Energy Diagram. At a turning point, the potential energy equals the mechanical energy and the kinetic energy is zero, indicating that the...
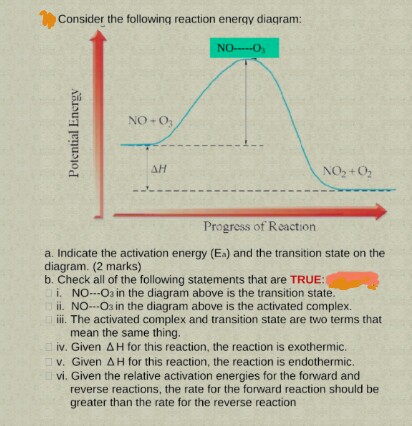
Activated complex maximum energy is reached. Activated complex an intermediate structure formed in the conversion of reactants to products. At the peak of the activation energy hump the reactants are in the transition state halfway between being reactants and forming products.
The transition state (or activated) complex is the species that lies at the top of the energy barrier on the reaction coordinate that converts reactants to products in a chemical process. For example, in an SN2 reaction the transition state complex is one in which five groups or atoms are transiently...
Click here to sign in with. On the heels of COP-26, where global leaders agreed to make unprecedented investments in the energy transition, frontline communities already in the crosshairs of mining for critical minerals warn of the dangers posed by the mining boom for 'green tech."
The energy required to reach the activated state must be available if the molecules are to change into where C#=K#ABCdenotes the concentrations of activated complexes. The rate of the reaction is then where E0is an energetic difference between the energy of activated state (product) and...
...that represents the activated complex (transition state) Identify the point Answer Bank activated complex Reaction Progress Energy (kJ/mol).
The energy systems will become even more diverse in the future therefore we will need more intelligent control systems. As a result of the WinReNN project subsidised by the German Federal Ministry for the Environment (BMUB), ZSW has been running an operational wind power forecasting system in...
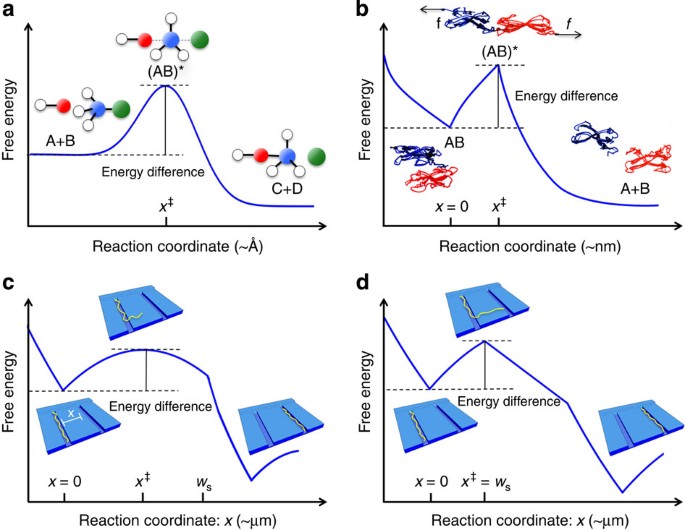
Transition State Theory Demonstrated At The Micron Scale With Out Of Equilibrium Transport In A Confined Environment Nature Communications
The Eyring activated-complex (or transition-state) treatment relates the observed rate constant k to The energy difference between the reactants and the transition state is the activation energy for At this point, rearrangement of bond lengths and bond angles in the two reactants, and of the...
Transition State = The Point Of Maximum Pain. In the Harry Potter series, Remus Lupin changed to his werewolf form when the moon was full. So given a diagram for the progress of a reaction, you can figure out the activation energy by subtracting the energy of the transition state from the...
Learn how clean energy transition and decarbonization are driving change in the energy and utilities sectors and leading toward a carbon-neutral future. Below are some of the most common renewable energy sources and the pros and cons their Net-zero emissions goals in a complex utilities market.
Phase diagram is a graphical representation of the physical states of a substance under different A phase transition is the transition from one state of matter to another. There are three states of Imagine a substance with the following points on the phase diagram: a triple point at .5 atm and -5°...
Click hereto get an answer to your question Energy of the activated complex is.activated complex is measured from the very bottom of the diagram to the top of the activation energy.On adding 0.095mol of Hg(CN)2 the freezing point of solution was −0.53∘C Assuming that complex is...
transition-state theory, also called activated-complex theory or theory of absolute reaction rates, treatment of chemical reactions and other processes that The difference between the energies of the transition and the initial states is closely related to the experimental activation energy for the...

Chemical Kinetics In Kinetics We Study The Rate At Which A Chemical Process Occurs Besides Information About The Speed At Which Reactions Occur Kinetics Ppt Download

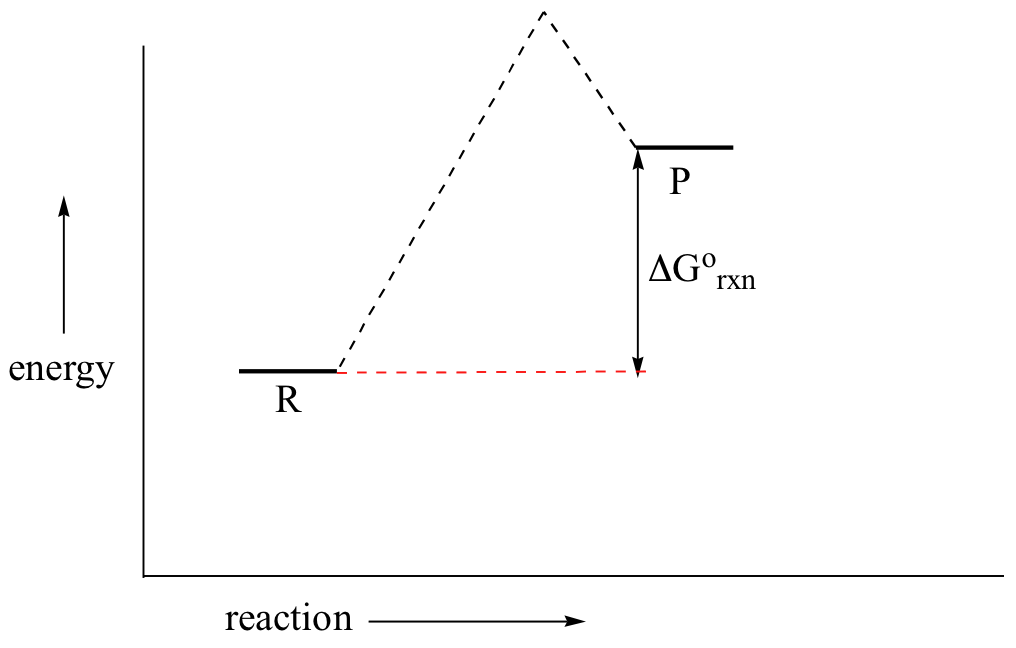
Draw An Energy Diagram For An Exothermic Reaction Label The Activation Enthalpy And The Change In Enthalpy Delta H On The Diagram Study Com
Solved Chapter 4 Problem 35sp Solution Organic Chemistry Plus Masteringchemistry With Etext Access Card Package 9th Edition Chegg Com

1chemistry 2c Lecture 22 May 21 Th Arrhenius Equation 2 Transition State Theory 3 Molecularity 4 Rate Limiting Steps 5 Reaction Mechanisms 6 Catalysis Ppt Download
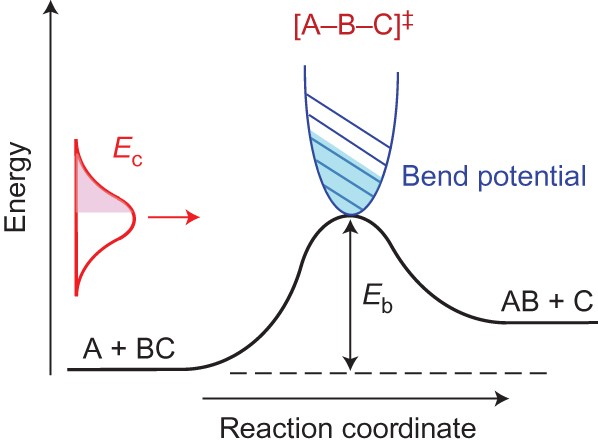
Direct Mapping Of The Angle Dependent Barrier To Reaction For Cl Chd3 Using Polarized Scattering Data Nature Chemistry

















0 Response to "40 click on the point of the energy diagram that represents the activated complex (transition state)."
Post a Comment