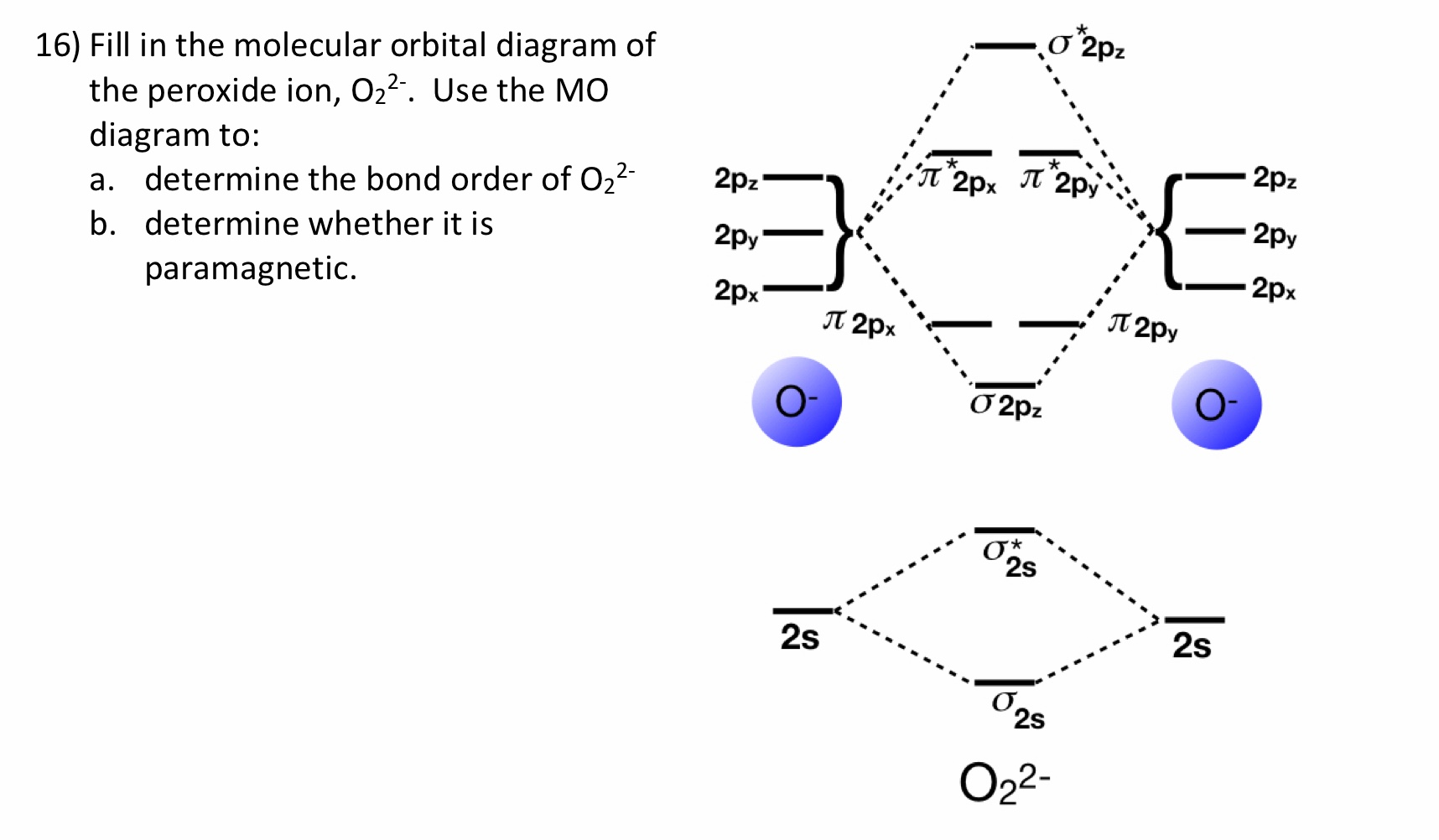
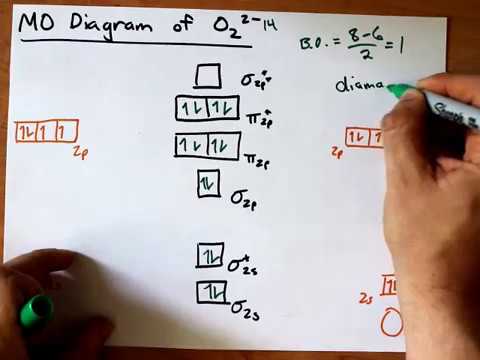
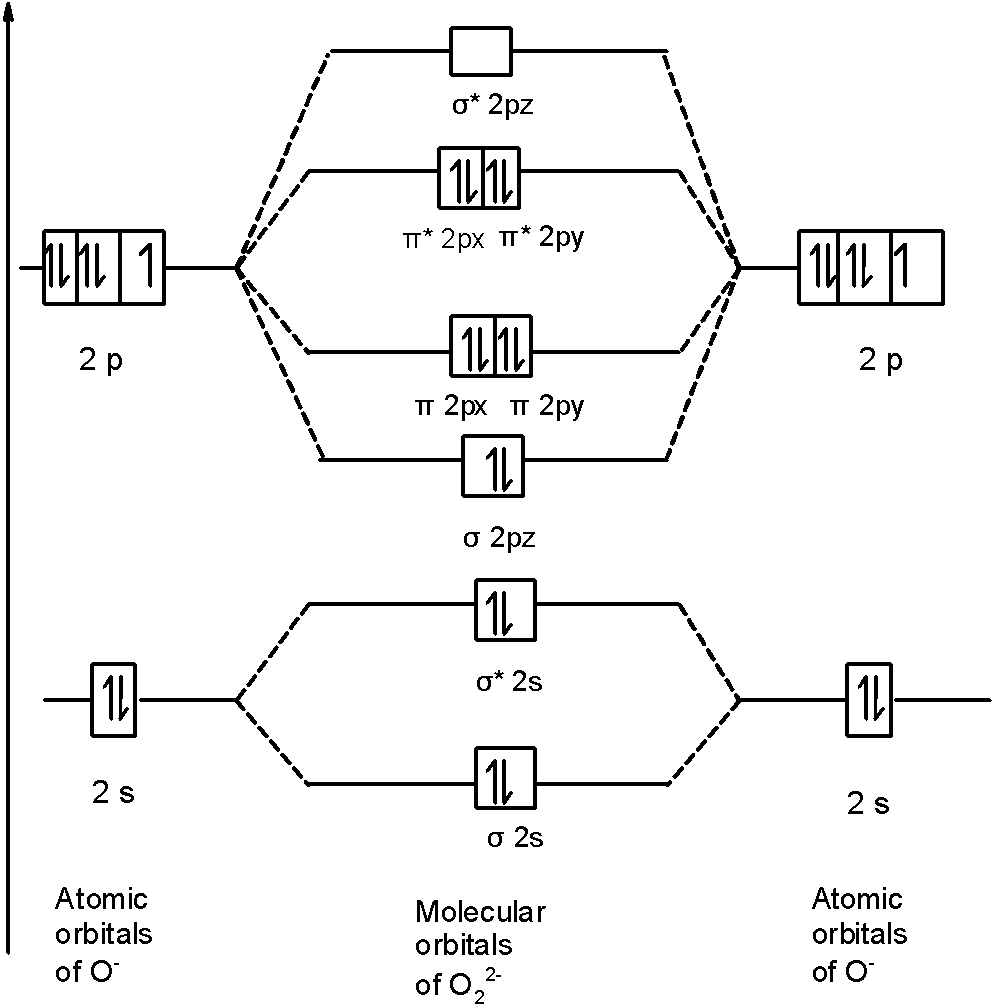
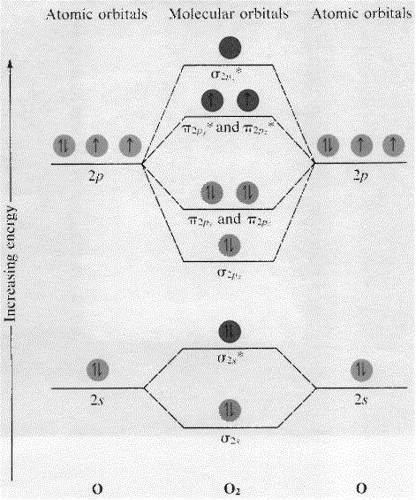
39 molecular orbital diagram for o2 2-
1 Answer. To draw a molecular orbital (MO) diagram, you need to consider which atomic orbitals (AOs) the molecule has. Oxygen atom is on period 2, so it has access to its 1s, 2s, and 2p AOs. Their relative energies are 2p > 2s >> 1s. (The 1s is much, much lower in energy than the 2s, and usually is not even on the MO diagram if done to-scale). This is the peroxide ion, O2(2-), so you KNOW it's going to be stable.It has a bond order of 1, which also makes sense. Draw the Lewis diagram of hydrogen pe...
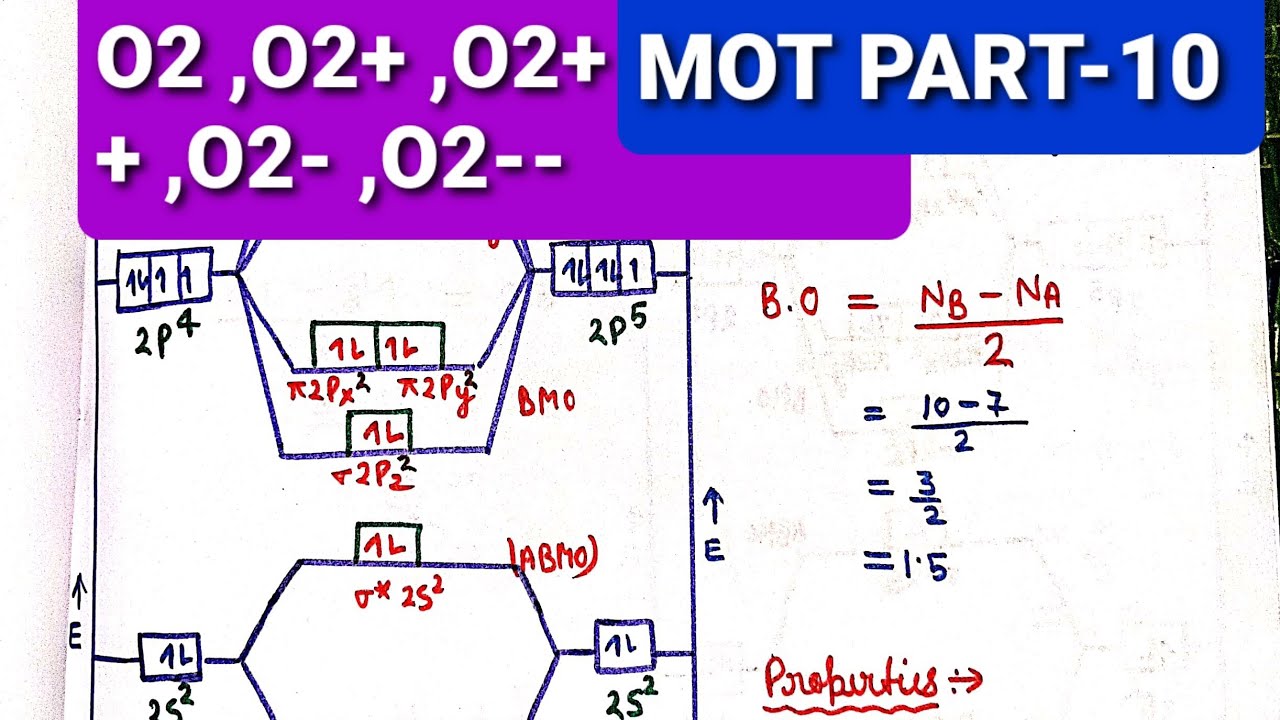
In this video, you will study about Molecular Orbital diagram of O2, O2+, O2(2-). We will also calculate the Bond order in each case and also the magnetic be...
Molecular orbital diagram for o2 2-
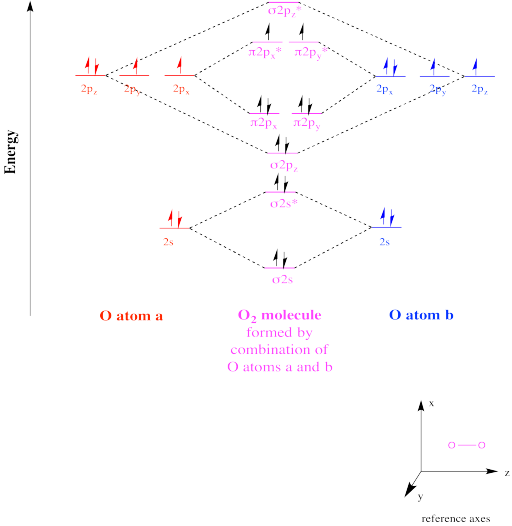
Draw the complete molecular orbital diagram for O2 , O2 - , and O2 2- . Using these diagrams, determine for each molecule the number of unpaired electrons, if they are paramagnetic or diamagnetic, and the bond order. Question: Draw the complete molecular orbital diagram for O2 , O2 - , and O2 2- . Using these diagrams, determine for each ... Answer (1 of 3): I modified the picture from this post: What's the MOT diagram of O2 +2 ion? and modified it to be O2 2+ (since sadly enough I am about as advanced with artistic programs on pc as a rock). How you basically do these questions is by first drawing the empty AO and MO, then counting ... O2 molecular orbital diagram oxygen has a similar setup to h 2 but now we consider 2s and 2p orbitals. This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals.
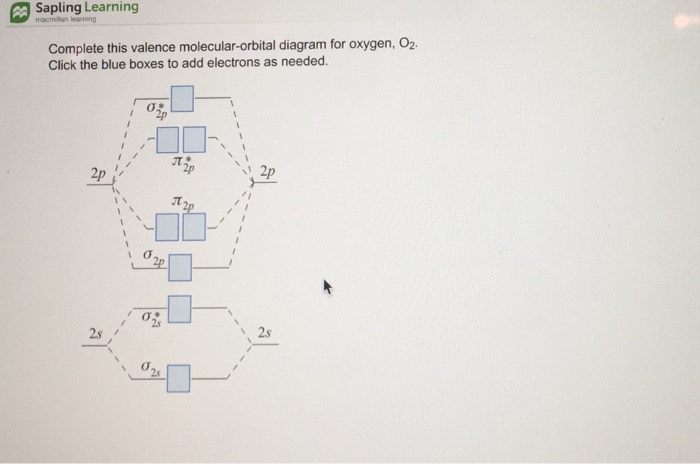
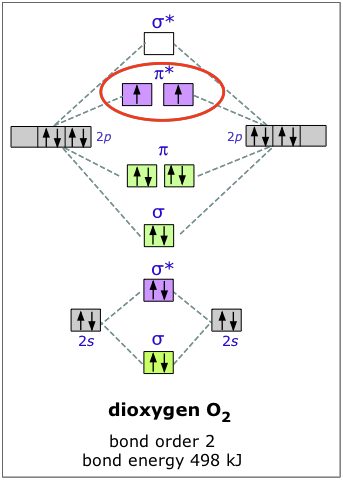
Molecular orbital diagram for o2 2-. For Free best Chemistry Notes, visit here👉 https://digitalkemistry.wordpress.com/For more informative Chemistry Lessons !!Subscribe 👉 https://www.youtube... Remember: When two oxygen atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals. They are flipped compare... Molecular orbital (MO) theory explains the construction of molecular orbital diagram on the basis of following main points . 1.Formation of MOs: Atomic orbitals(AOs) linearly combine with each other to form equal number of molecular orbitals (MOs). 2.Energy of MOs: Half of the molecular orbitals (MOs) having energy lower than the atomic orbitals are called… Solution. The bond size in the oxygen varieties can be explained by the positions of the electrons in molecular orbital theory. To achieve the molecular orbit energy-level diagram because that \ (\ceO2\), we need to ar 12 valence electrons (6 from each O atom) in the energy-level diagram presented in number \ (\PageIndex1\).
O2 molecular orbital diagram oxygen has a similar setup to h 2 but now we consider 2s and 2p orbitals. This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals. Answer (1 of 3): I modified the picture from this post: What's the MOT diagram of O2 +2 ion? and modified it to be O2 2+ (since sadly enough I am about as advanced with artistic programs on pc as a rock). How you basically do these questions is by first drawing the empty AO and MO, then counting ... Draw the complete molecular orbital diagram for O2 , O2 - , and O2 2- . Using these diagrams, determine for each molecule the number of unpaired electrons, if they are paramagnetic or diamagnetic, and the bond order. Question: Draw the complete molecular orbital diagram for O2 , O2 - , and O2 2- . Using these diagrams, determine for each ...

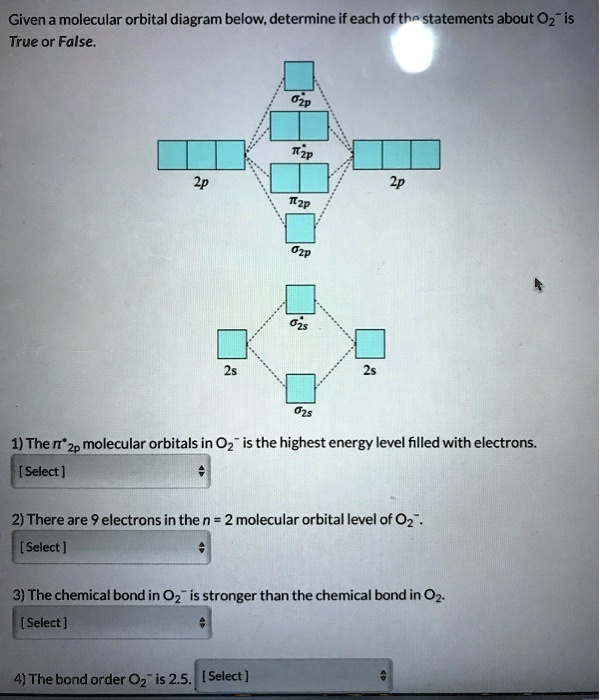
Solved Given A Molecular Orbital Diagram Below Determine If Each Of The Statements About 02 Is True Or False 1 The It 2p Molecular Orbitals In 02 Is The Highest Energy Level Filled With

Draw Molecular Orbital Energy Diagram Of O2 Molecule And Mention Its Bond Order Explain Why O2 Brainly In
Draw The M O Diagram For Oxygen Molecule And Calculate Its Bond Order And Show That O2 Is Paramagnetic Sarthaks Econnect Largest Online Education Community
Using Molecular Orbital Theory Compare The Bond Energy And Magnetic Character Of O2 And O2 Species Sarthaks Econnect Largest Online Education Community
Molecular Orbital Theory Chemistry Encyclopedia Structure Number Molecule Atom Bond Order Multiple Bonds

Pleaseshow Me The Energy Level Diagrams Of O2 O2 O2 2 Chemistry Chemical Bonding And Molecular Structure 7027301 Meritnation Com

Draw A Molecular Orbital Energy Level Diagram For O2 And No Compare And Contrast The Formation Homeworklib

Based On The Mo Diagrams For O 2 O 2 And O 2 Answer The Following 1 Is O 2 Paramagnetic Or Diamagnetic 2 Which Will Have The Shortest Bond Length 3 Which Will Have The

Consider The Molecular Orbital Diagram For The Ion O 2 2 Predict The Bond Order A 3 0 B 2 5 C 1 0 D 2 0 E 1 5 Consider The Following Statements Will The Ion Be Paramagnetic Or Study Com

















0 Response to "39 molecular orbital diagram for o2 2-"
Post a Comment