39 lever rule phase diagram
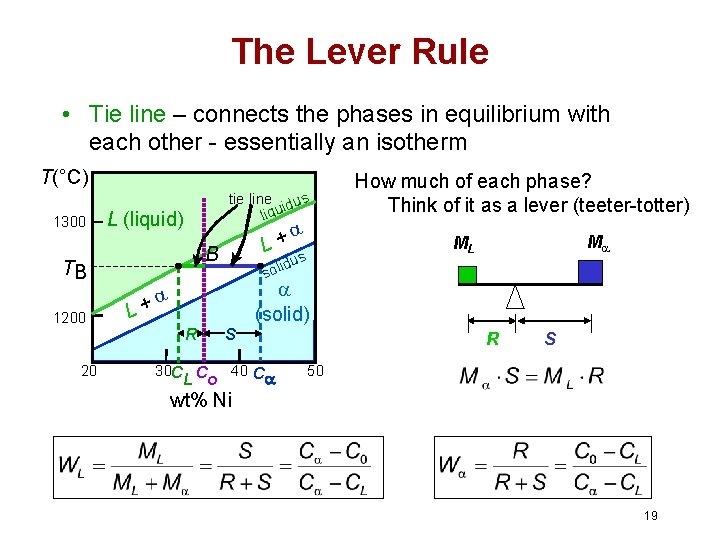
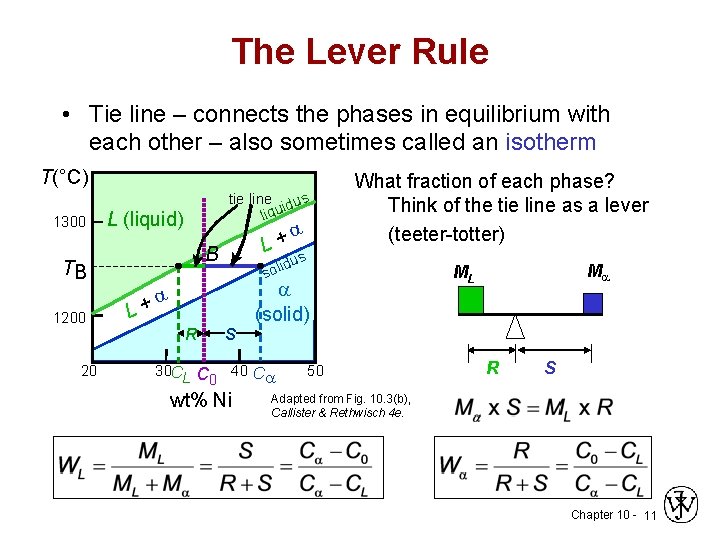
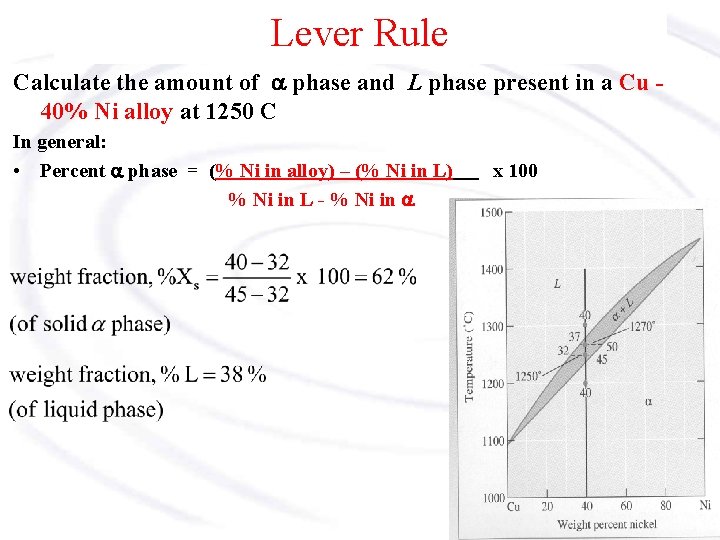
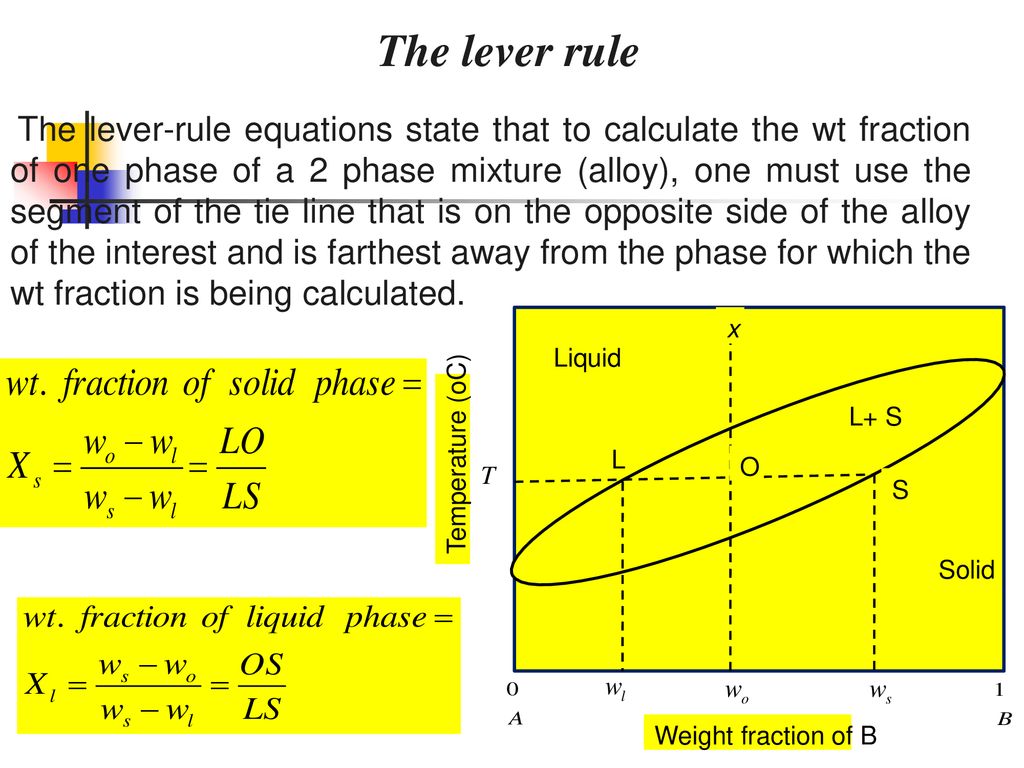
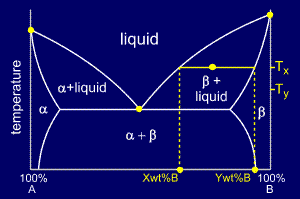
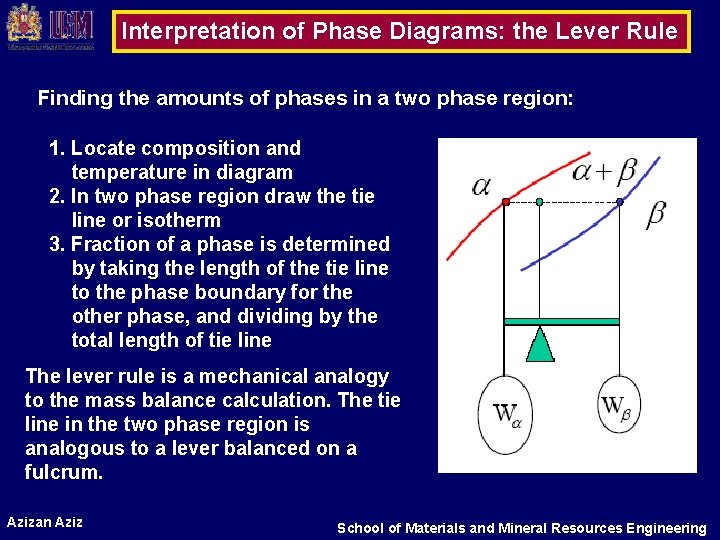
MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 15 The lever rule Finding the amounts of phases in a two phase region: 1. Locate composition and temperature in diagram 2. In two phase region draw the tie line or isotherm 3. Fraction of a phase is determined by taking the length of the tie line to the phase boundary for the ... Chapter 8 10 8.5 The Lever Rule The weight percentages of the phases in any 2 phase region can be calculated by using the lever rule Let x be the alloy composition of interest, its mass fraction of B (in A) is Cο Let T be the temperature of interest ⇒at T alloy x consists of a mixture of liquid (with C L-mass fraction of B in liquid) and solid (C S - mass fraction of B in solid phase)
can be determined by the lever rule). This looks a lot like equation (2), if we simply notice that in the case of the pure components, the mole fraction of A is X. A. and of B is X. B. Notice also that the line connecting points (4) and (5) acts like the line in the plot of the gibbs free energy of the . pure, combined. components as a function of composition. For example, let’s say we had a ...
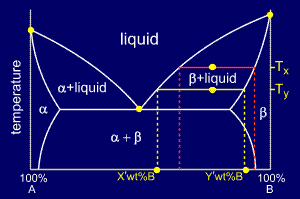
Lever rule phase diagram
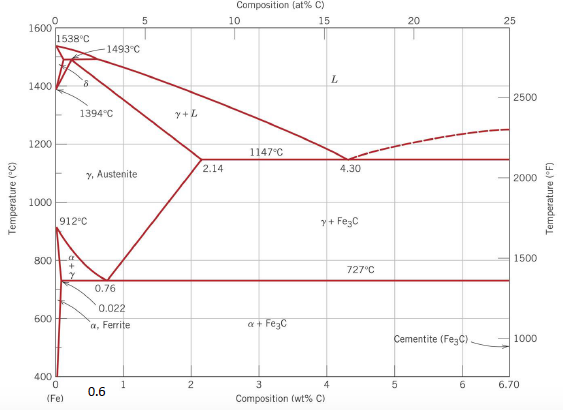
1:13Demonstrate how to use the lever rule on a Txy diagram. Made by faculty at the University of Colorado ...23 Nov 2011 · Uploaded by LearnChemE We are given a hypothetical eutectic phase diagram for which Ceutectic = 47 wt% B, Cβ = 92.6 wt% B at the eutectic temperature, and also that Wα' = 0.356 and Wα = 0.693; from this we are asked to determine the composition of the alloy. Let us write lever rule expressions for Wα' and Wα Wα= CβŹ− C0 Cβ−Cα = 92.6 − C0 92.6 − Cα ... QUESTIONS Diff. between; (i) Phase rule and Phase diagram (ii) Solvus line and Solidus line What is phase? Explain invariant reaction with example. Explain lever rule with a tie line Expalin all transformation reaction.(p64) Explain eutectoid and eutectic reaction in Fe-C binary phase diagram (p45)
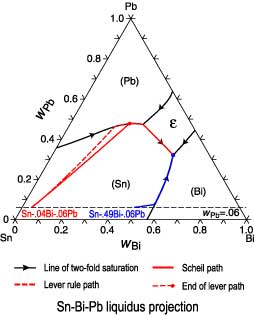
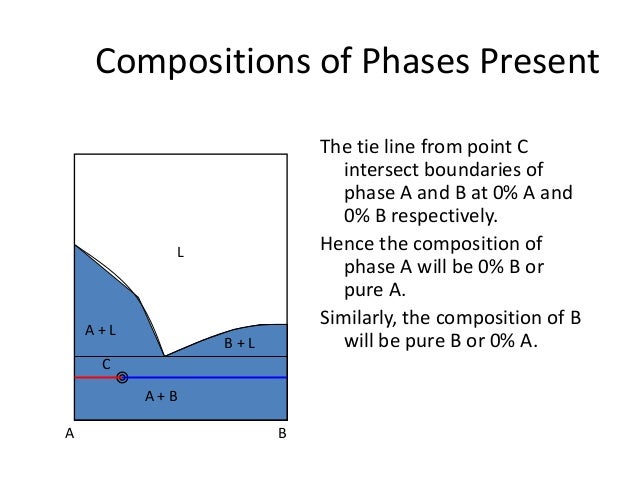
Lever rule phase diagram. In thermodynamics, the phase rule is a general principle governing "pVT" systems (that is, systems whose states are completely described by the variables pressure (p), volume (V) and temperature (T)) in thermodynamic equilibrium.If F is the number of degrees of freedom, C is the number of components and P is the number of phases, then = + It was derived by American physicist Josiah Willard ... As explained in Sec. 8.2, a phase diagram is a kind of two-dimensional map that shows which phase or phases are stable under a given set of conditions. This section discusses some common kinds of binary systems, and Sec. 13.3 will describe some interesting ternary systems. 13.2.1 Generalities. A binary system has two components; \(C\) equals \(2\), and the number of degrees of freedom is \(F=4 ... At first glance, there seems to be a lot going on in a two-phase diagram. There are temperatures, percentages, different elements, and symbols you’re not used to seeing. But once you understand what you’re looking for, everything makes sense. Here is an example of a phase diagram for an SiO₂ and Al₂O₃ mixture. On the x axis, we have the mole % of Al₂O₃ in the mixture. Ternary phase diagrams are used to represent all possible mixtures of three solvents [1]; they are described in Chapter 3.Here, we shall indicate how they should be used to minimize the solvent consumption. Figure 2.1 (top) shows the methanol–chloroform–water ternary phase diagram with the tie-lines in the biphasic domain. Five particular compositions are shown in the diagram: Table 2.1 ...
7:19Applies the lever rule to a solid-liquid mixture to determine the fraction of each phase in equilibrium and ...13 Nov 2011 · Uploaded by LearnChemE 26 Nov 2020 — If an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever rule to the phase diagram. Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course: -binary systems: just 2 components. -independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys, P. Nash Tie line –Lever rule At a point in a phase diagram, phases present and their composition (tie-line method) along with relative fraction of phases (lever rule) can be computed. Procedure to find equilibrium concentrations of phases (refer to the figure in previous slide): - A tie-line or isotherm (UV) is drawn across two-phase region to intersect the boundaries of the region. - Perpendiculars ...
In chemistry, the lever rule is a formula used to determine the mole fraction (x i) or the mass fraction (w i) of each phase of a binary equilibrium phase diagram.It can be used to determine the fraction of liquid and solid phases for a given binary composition and temperature that is between the liquidus and solidus line.. In an alloy or a mixture with two phases, α and β, which themselves ... the phase diagram. Here we must use the lever rule to calculate the mass fraction of each phase. Unfortunately, figure 10.2 does not contain an insert enlarging the α+L region of the phase diagram, so accurate reading of this figure is difficult. However, if we draw a tie line 5:00AboutPressCopyrightContact usCreatorsAdvertiseDevelopersTermsPrivacyPolicy & SafetyHow YouTube ...21 May 2018 · Uploaded by Taylor Sparks Sometimes it is also known as the “reverse arm rule,” because for the calculation of α L (liquid) you use the “arm” within the (y A -x A) segment closest to the vapor, and for the vapor calculation (α G) you use the “arm” closest to the liquid. Figure 5.5: The Lever Rule In a P-x Diagram. At this point you will see clearly why we ...
A point in the two-phase region of a phase diagram indicates not only qualitatively that the liquid and vapor are present, but represents quantitatively the relative amounts of each. To find the relative amounts of two phases a & b in equilibrium, we measure distances on the tie line, l a and l b between the two phases and use the lever rule:
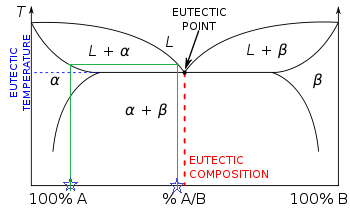
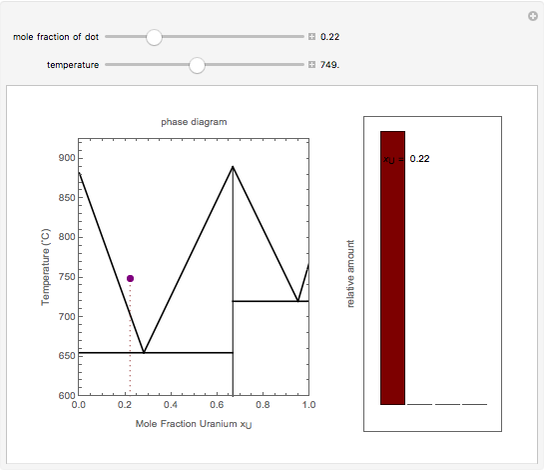
There relative amount has to follow the lever rule, which is just a linear variation across the two-phase region from, say, all γ right on the boundary of γ with γ + δ, to all δ on the other side. The horizontal lines are there mostly to emphasize when a new phase appears or disappears. So, at point E on you diagram above, there is one ...
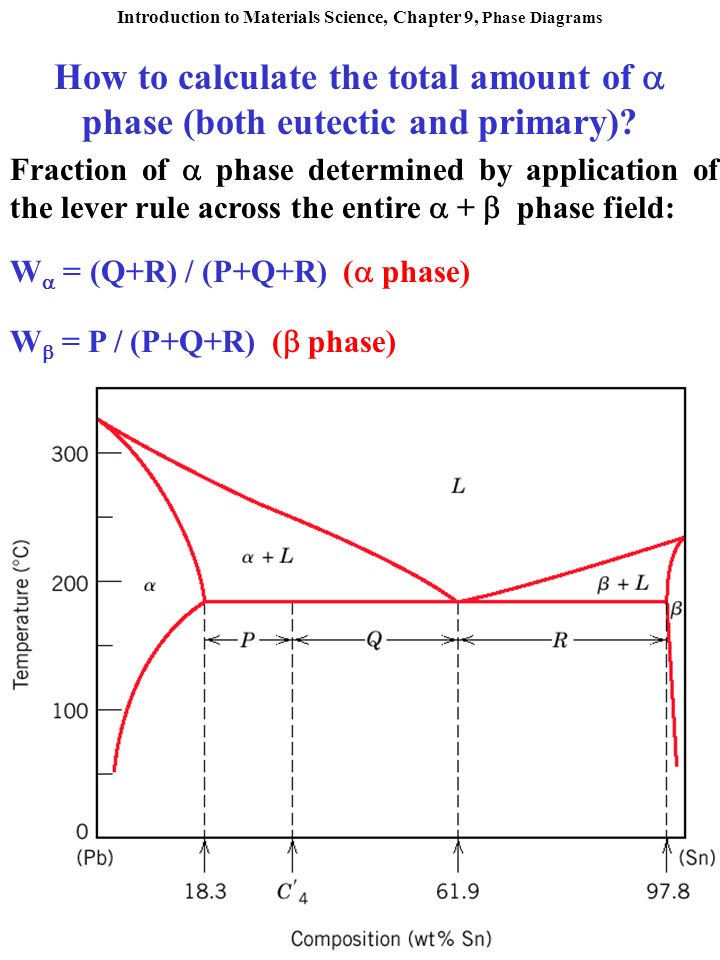
How To Calculate The Total Amount Of Phase Both Eutectic And Primary Fraction Of Phase Determined By Application Of The Lever Rule Across The Entire Ppt Video Online Download
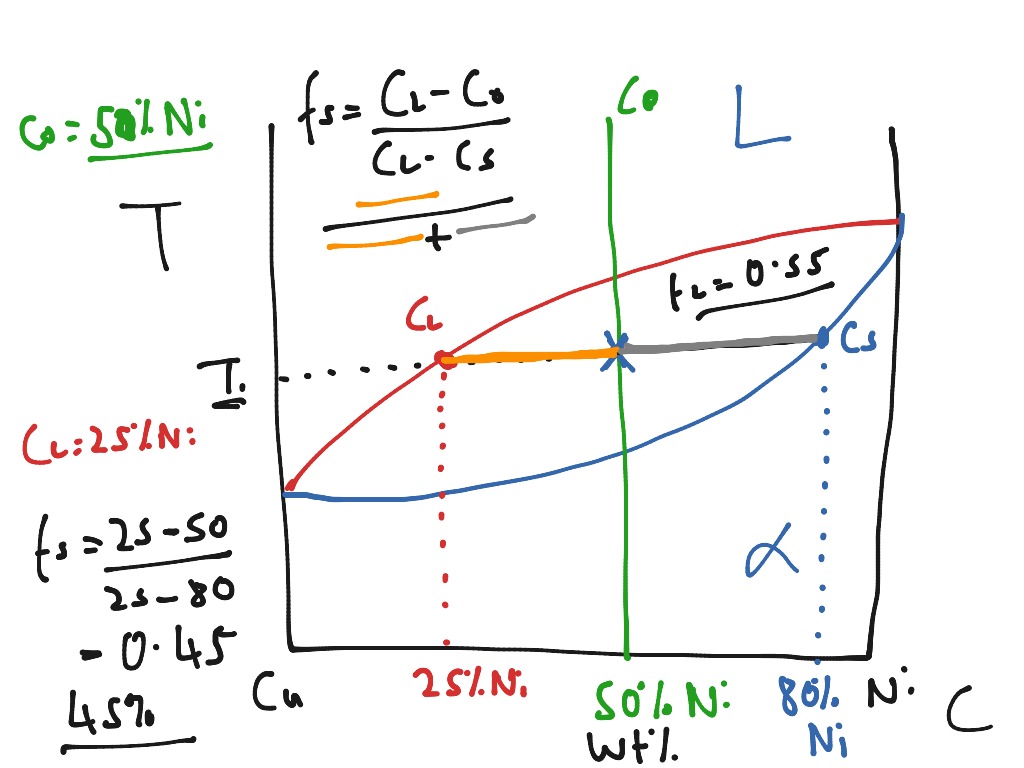
As shown on the phase diagram, the alloy is, at the given temperature, a mixture of alpha and liquid phases - but what are their exact compositions at this ...
If an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever rule to the phase diagram. The lever rule ...
• Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash (Ed.), ASM International, Materials Park, OH (1991). • 2 phases: L (liquid) α (FCC solid solution) • 3 phase fields: L L + α α 0 20 40 60 80 100 wt% Ni 1000 1100 1200 1300 1400 1500 1600 T(°C) L (liquid) α (FCC solid solution) L + α ...
5:12Phase diagrams contain two-phase regions. We can use the lever rule to determine the phase fraction of ...3 Sep 2020 · Uploaded by Taylor Sparks
14:02Video tutorial illustrating application and derivation of the lever rule in a simple isomorphous binary phase ...19 Dec 2014 · Uploaded by Patrick Shamberger
• The phase diagram provides limits to how high the concentrations, Cl or Cs can rise due to segregation. Second phase formation (generally from eutectic reaction) then occurs. Gulliver–Scheil is independent of the shape of the S/L interface! Where χ s= x L (1−χ s) (k 0−1) = C l C 0
QUESTIONS Diff. between; (i) Phase rule and Phase diagram (ii) Solvus line and Solidus line What is phase? Explain invariant reaction with example. Explain lever rule with a tie line Expalin all transformation reaction.(p64) Explain eutectoid and eutectic reaction in Fe-C binary phase diagram (p45)
We are given a hypothetical eutectic phase diagram for which Ceutectic = 47 wt% B, Cβ = 92.6 wt% B at the eutectic temperature, and also that Wα' = 0.356 and Wα = 0.693; from this we are asked to determine the composition of the alloy. Let us write lever rule expressions for Wα' and Wα Wα= CβŹ− C0 Cβ−Cα = 92.6 − C0 92.6 − Cα ...
1:13Demonstrate how to use the lever rule on a Txy diagram. Made by faculty at the University of Colorado ...23 Nov 2011 · Uploaded by LearnChemE


















0 Response to "39 lever rule phase diagram"
Post a Comment