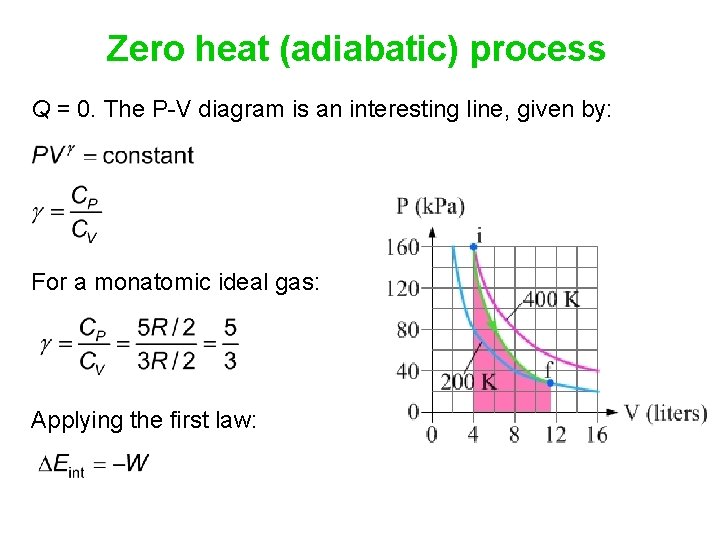
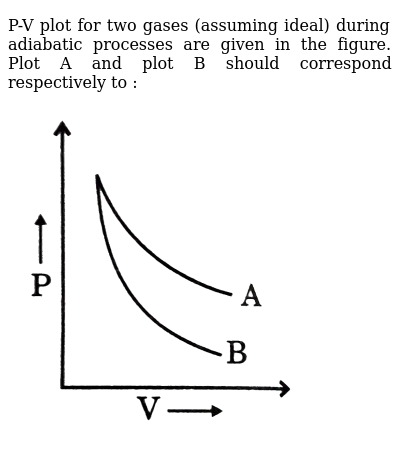
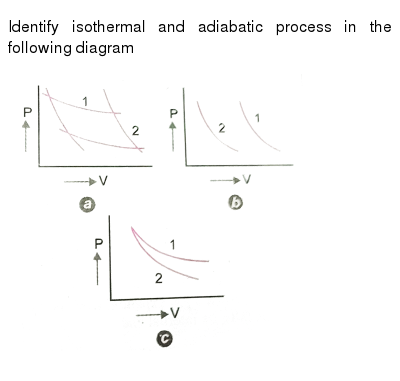
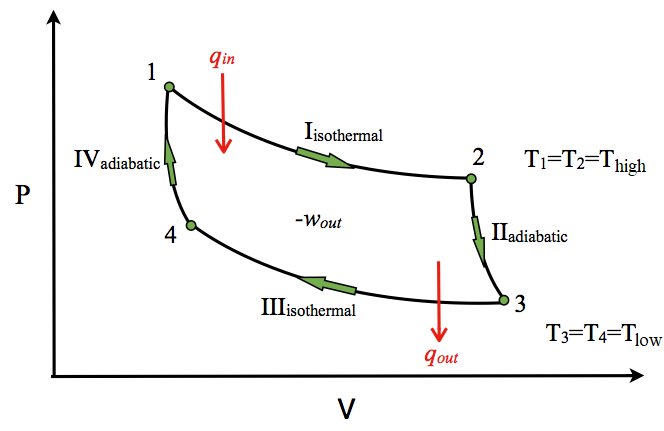
39 adiabatic process pv diagram
During an adiabatic process no heat is transferred to the gas, but the temperature, pressure, and volume of the gas change as shown by the dashed line. As ... If the system is allowed to expand, doing work, the energy comes from the internal energy - the gas must cool. The P-V diagram for this process shows the ...
The PV Diagram Shows The Cycle Of A Diatomic Gas I ... Bridget's Chemistry Blog: Molar Mass; ... n moles of a diatomic gas has undergone a cyclic process ... A diatomic gas undergoes adiabatic changes. What is its ... How do I solve this? ...
Adiabatic process pv diagram
An adiabatic process is one in which no heat is gained or lost by the system. The first law of thermodynamics with Q=0 shows that all the change in internal ... On a p-V diagram, an adiabatic process occurs along a line (called an adiabat) that has the equation p = constant / Vκ. Adiabatic Curve - Adiabat. Zhaoqi Ji, Jianuo Chen, María Pérez-Page, Zunmin Guo, Ziyu Zhao, Rongsheng Cai, Maxwell T. P. Rigby, Sarah J. Haigh, Stuart M. Homes. Nitrogen doping of the carbon is an important method to improve the performance and durability of catalysts for proton exchange membrane fuel cells by platinum-nitrogen and carbon-nitrogen bonds....
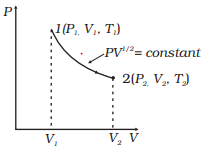
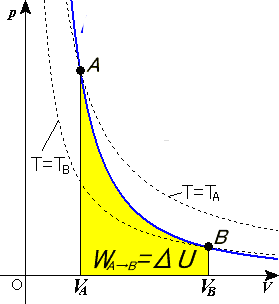
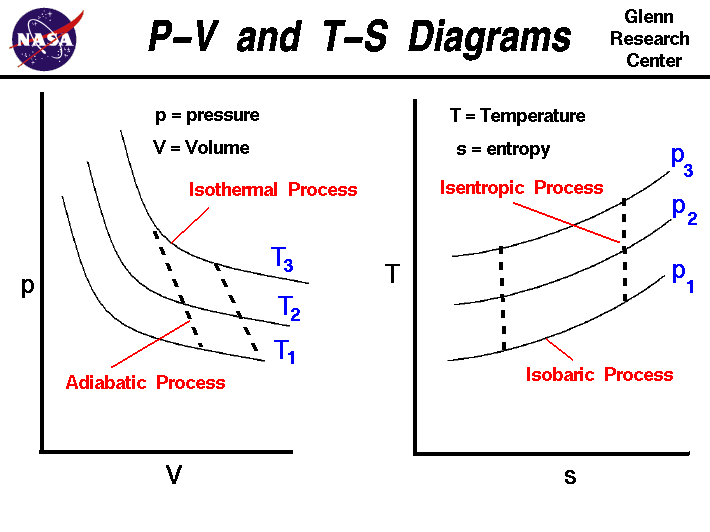
Adiabatic process pv diagram. The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. For an ideal gas, an adiabatic process is a reversible process with constant entropy. The mathematical representation of the adiabatic process is ΔQ=0 Process 1-2: Reversible Adiabatic Compression Process: The cylinder contains full of air which is entered through the inlet port as we studied above. Here P1, V1, and T1 are the corresponding Pressure, Volume, and Temperature. After the adiabatic compression process in which entropy remains constant and the air is compressed by the piston. The PV diagram for an adiabatic process show a special result. • An adiabatic process looks very much like an isothermal process, but it drops off to a lower point. This means it is on a different isotherm. Remember, isotherms are just lines that show where the temperature stays constant. Sep 08, 2019 · Process 4-1 Isentropic Compression. Now again the insulated cap I.C. is brought in contact with the bottom of the cylinder B, and the air is allowed to be compressed adiabatically. The adiabatic compression is represented by the curve 4-1 on p-v and T-s diagram. The temperature of the air increases from T4 to T1. Since no heat is absorbed or ...
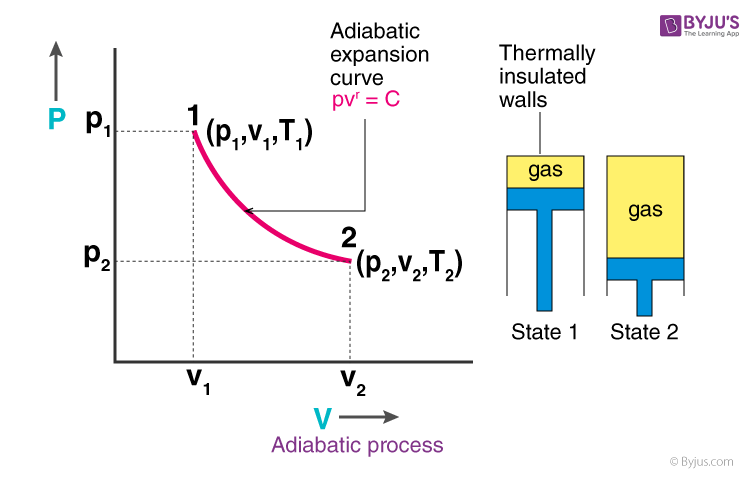
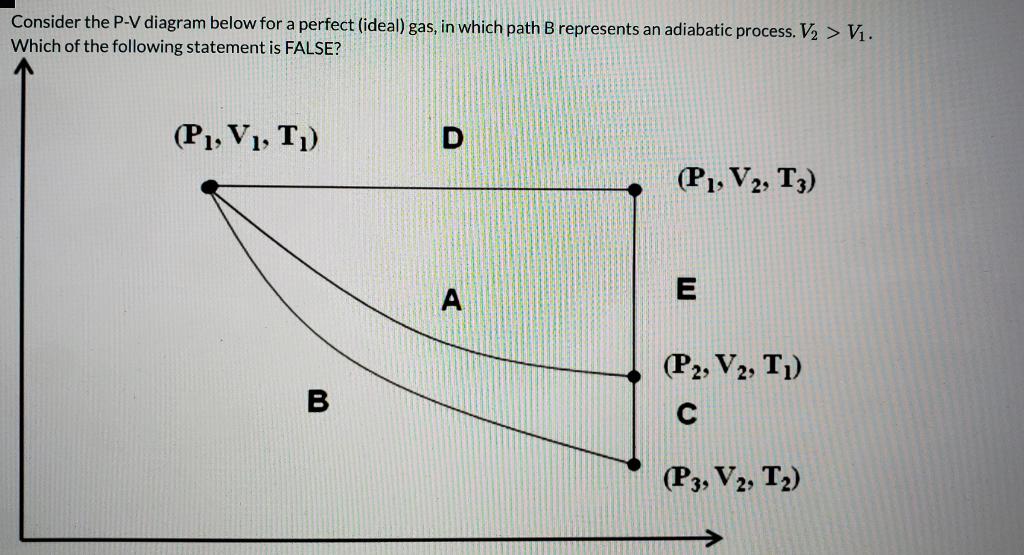
A reversible adiabatic expansion of an ideal gas is represented on the pV diagram of (Figure). The slope of the curve at any point is. SBOBET is a website that provides all kinds of legal sports betting services. At present, there are many open web sites that are networked from the main web or online agents. Whic May 22, 2019 · Adiabatic Process. An adiabatic process is a thermodynamic process, in which there is no heat transfer into or out of the system (Q = 0). The system can be considered to be perfectly insulated.In an adiabatic process, energy is transferred only as work. The assumption of no heat transfer is very important, since we can use the adiabatic approximation only in very rapid processes. In thermodynamics, an isentropic process is an idealized thermodynamic process that is both adiabatic and reversible. The work transfers of the system are frictionless, and there is no net transfer of heat or matter. Such an idealized process is useful in engineering as a model of and basis of comparison for real processes. This is idealized as reversible processes do not occur in reality ...
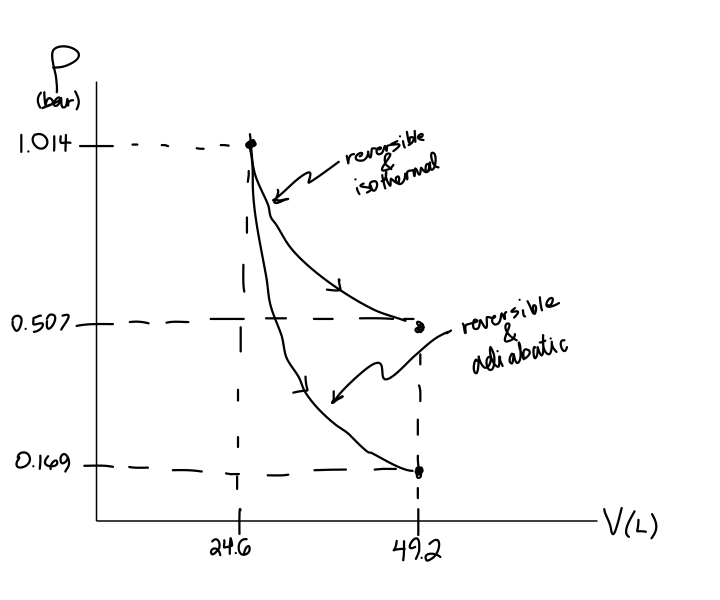
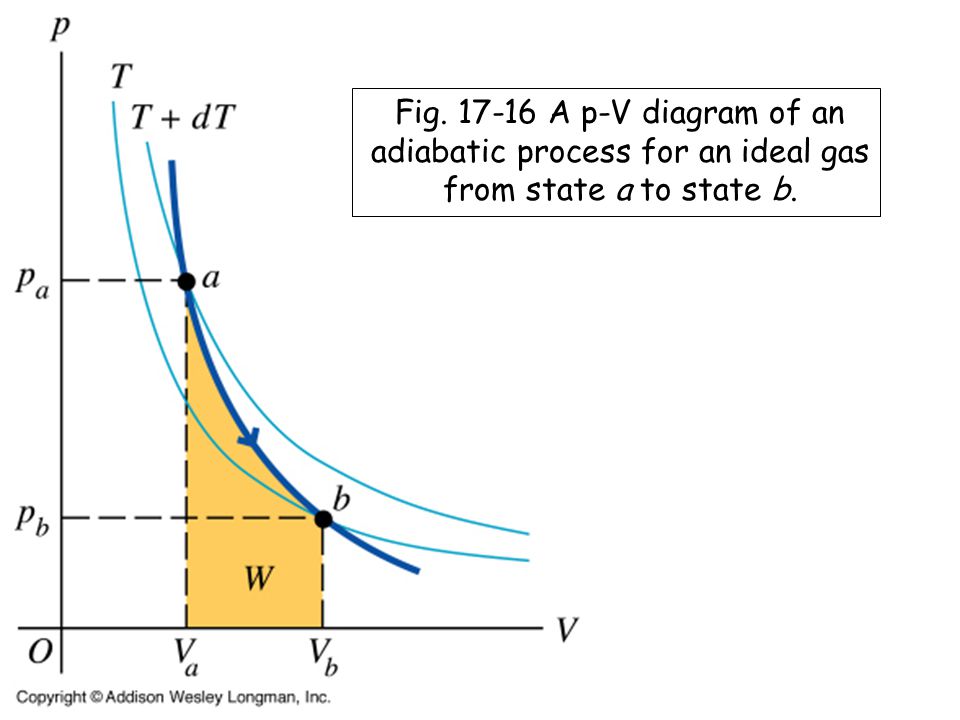
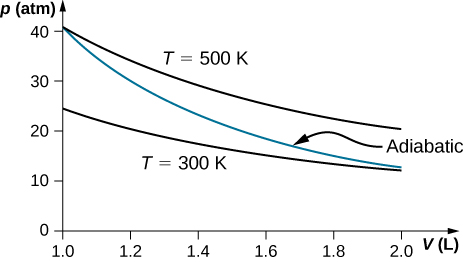
Nov 08, 2021 · Figure \(\PageIndex{3}\): Quasi-static adiabatic and isothermal expansions of an ideal gas. The dashed curve shown on this pV diagram represents an isothermal expansion where \(T\) (and therefore pV) is constant. The slope of this curve is useful when we consider the second law of thermodynamics in the next chapter. This slope is May 22, 2019 · Adiabatic Process. An adiabatic process is a thermodynamic process, in which there is no heat transfer into or out of the system (Q = 0). The system can be considered to be perfectly insulated.In an adiabatic process, energy is transferred only as work. The assumption of no heat transfer is very important, since we can use the adiabatic approximation only in very rapid processes. pV γ pVγ 1 constant 2 2 1 1 1 = = T Vγ− T V γ− pV =nRT During an adiabatic expansion process, the reduction of the internal energy is used by the system to do work on the environment. During an adiabatic compression process, the environment does work on the system and increases the internal energy. Ideal gas: adiabatic process (contd) Figure 5 shows a graph of pressure versus volume (that is, a PV diagram for an ... Both isothermal and adiabatic processes such as shown in Figure 8 are ...
The mathematical equation for an ideal gas undergoing a reversible (i.e., no entropy generation) adiabatic process can be represented by the polytropic process equation =, where P is pressure, V is volume, and for this case n = γ, where = = +, C P being the specific heat for constant pressure, C V being the specific heat for constant volume, γ is the adiabatic index, and f is the number of ...
Zhaoqi Ji, Jianuo Chen, María Pérez-Page, Zunmin Guo, Ziyu Zhao, Rongsheng Cai, Maxwell T. P. Rigby, Sarah J. Haigh, Stuart M. Homes. Nitrogen doping of the carbon is an important method to improve the performance and durability of catalysts for proton exchange membrane fuel cells by platinum-nitrogen and carbon-nitrogen bonds....
On a p-V diagram, an adiabatic process occurs along a line (called an adiabat) that has the equation p = constant / Vκ. Adiabatic Curve - Adiabat.
An adiabatic process is one in which no heat is gained or lost by the system. The first law of thermodynamics with Q=0 shows that all the change in internal ...

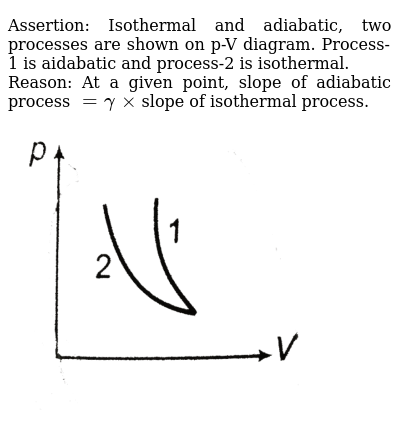












0 Response to "39 adiabatic process pv diagram"
Post a Comment